Sandbox Reserved 988
From Proteopedia
| Line 6: | Line 6: | ||
== Background == | == Background == | ||
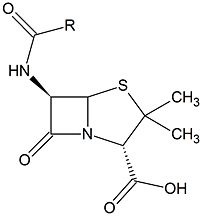
| - | [[Image:Beta-lactam.jpg|200px|thumb|right|A [http://en.m.wikipedia.org/wiki/Beta-lactam_antibiotic β-lactam antibiotic] ([http://en.m.wikipedia.org/wiki/Penicillin Penicillin])]]Clinically, [http://en.m.wikipedia.org/wiki/Beta-lactam_antibiotic β-lactam antibiotics], characterized by their central chemical structure, are utilized to combat bacterial infections by targeting [http://proteopedia.org/wiki/index.php/Penicillin-binding_protein penicillin-binding proteins] (PBPs), also known as [http://en.m.wikipedia.org/wiki/DD-transpeptidase transpeptidases]. PBPs are enzymes that are located in the cell membrane of bacteria and function in [http://en.wikipedia.org/wiki/Cross-link cross-linking] to form the [http://en.m.wikipedia.org/wiki/Peptidoglycan peptidoglycan] layer | + | [[Image:Beta-lactam.jpg|200px|thumb|right|A [http://en.m.wikipedia.org/wiki/Beta-lactam_antibiotic β-lactam antibiotic] ([http://en.m.wikipedia.org/wiki/Penicillin Penicillin])]]Clinically, [http://en.m.wikipedia.org/wiki/Beta-lactam_antibiotic β-lactam antibiotics], characterized by their central chemical structure, are utilized to combat bacterial infections by targeting [http://proteopedia.org/wiki/index.php/Penicillin-binding_protein penicillin-binding proteins] (PBPs), also known as [http://en.m.wikipedia.org/wiki/DD-transpeptidase transpeptidases]. PBPs are enzymes that are located in the cell membrane of bacteria and function in [http://en.wikipedia.org/wiki/Cross-link cross-linking] to form the [http://en.m.wikipedia.org/wiki/Peptidoglycan peptidoglycan] layer of the bacterial cell wall. <ref>"Peptidoglycan cell wall." The University of Warwick. n.d. Web. 25 Jan 15</ref> This cross-linking process strengthens the cell wall and enables the bacteria to resist osmotic pressure from its external environment. |
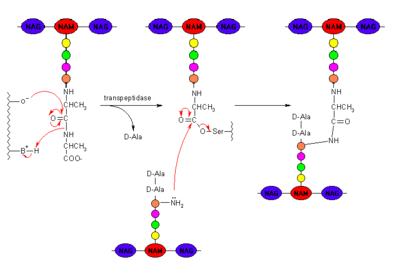
[[Image:Peptidoglycan_cross_linking.png|400px|thumb|center|Peptidoglycan with PBP Cross-linking Mechanism]] | [[Image:Peptidoglycan_cross_linking.png|400px|thumb|center|Peptidoglycan with PBP Cross-linking Mechanism]] | ||
| - | + | β-lactam antibiotics covalently bind to PBPs, [http://en.m.wikipedia.org/wiki/Enzyme_inhibitor inhibiting] them from executing their role in properly cross-linking the peptidoglycan layer of the cell wall. The catalytic serine in the active site of a PBP performs a nucleophilic attack on the carbonyl carbon of a β-lactam ring. The β-lactam cannot be removed and thus permanently renders the PBP incapable of its catalytic function. Ultimately, this results in death of bacterial cells from osmotic instability or [http://en.m.wikipedia.org/wiki/Autolysis_(biology) autolysis].<ref name="MSUDP 2014"> | |
Beta Lactam Antibiotics, 2011. Antimicrobial Resistance Learning Site. Michigan State University Department of Pharmacology. 16 Sept, 2014. | Beta Lactam Antibiotics, 2011. Antimicrobial Resistance Learning Site. Michigan State University Department of Pharmacology. 16 Sept, 2014. | ||
</ref> | </ref> | ||
| - | One of the main causes of [http://en.wikipedia.org/wiki/Antimicrobial_resistance resistance] to β-lactam | + | One of the main causes of [http://en.wikipedia.org/wiki/Antimicrobial_resistance resistance] to β-lactam antibiotics is caused by β-lactamases. Chemically, β-lactamases and PBPs bind to β-lactam antibiotics in similar mechanisms, nucleophilic attack of the enzyme's catalytic serine on to the antibiotics carbonyl group. However, β-lactamases are then able to deactivate the antimicrobial activity of the β-lactams by cleaving the β-lactam bound in the <scene name='69/691534/Class_c_beta-lactamase_as/2'>active site</scene><ref>Powers, Rachel, Hollister C. Swanson, Magdalena A. Taracila, Nicholas W. Florek, Chiara Romagnoli, Emilia Caselli, Fabio Prati, Robert A. Bonomo, and Bradley J. Wallar. Biochemical and Structural Analysis of Inhibitors Targeting the ADC-7 Cephalosporinase of Acinetobacter baumannii. Biochemistry, 2014, 53 (48), 7670-7679.</ref> through a molecular process called [http://en.m.wikipedia.org/wiki/Acetylation deacylation], rendering it incapable of inhibiting the PBPs and ultimately, allowing cross-linking to occur for adequate cell wall formation. |
== History == | == History == | ||
| - | Many medical textbooks recount Alexander Flemming’s serendipitous observation of the antibacterial action of Penicillium mold in 1928. While not the first to note the inhibitory effect, Flemming became the first to seriously push the scientific community to research the isolation of the active compound, which he named penicillin. Eventually these efforts paid off | + | Many medical textbooks recount Alexander Flemming’s serendipitous observation of the antibacterial action of Penicillium mold in 1928. While not the first to note the inhibitory effect, Flemming became the first to seriously push the scientific community to research the isolation of the active compound, which he named penicillin. Eventually these efforts paid off and in 1940, Sir Ernst Boris Chain published a method of isolating and purifying penicillin and tested its clinical effectiveness in mice. Later that year, he identified the first β-lactamase in Escherichia coli. While interesting, this discovery was not considered clinically relevant until β-lactamase enzymes were isolated from clinical samples of gram positive bacteria by Dr. William M. M. Kirby. |
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
Revision as of 19:01, 14 December 2015
| This Sandbox is Reserved from 20/01/2015, through 30/04/2016 for use in the course "CHM 463" taught by Mary Karpen at the Grand Valley State University. This reservation includes Sandbox Reserved 987 through Sandbox Reserved 996. |
To get started:
More help: Help:Editing |
Contents |
Your Heading Here (maybe something like 'Structure')
This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs. You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Background
Clinically, β-lactam antibiotics, characterized by their central chemical structure, are utilized to combat bacterial infections by targeting penicillin-binding proteins (PBPs), also known as transpeptidases. PBPs are enzymes that are located in the cell membrane of bacteria and function in cross-linking to form the peptidoglycan layer of the bacterial cell wall. [3] This cross-linking process strengthens the cell wall and enables the bacteria to resist osmotic pressure from its external environment.β-lactam antibiotics covalently bind to PBPs, inhibiting them from executing their role in properly cross-linking the peptidoglycan layer of the cell wall. The catalytic serine in the active site of a PBP performs a nucleophilic attack on the carbonyl carbon of a β-lactam ring. The β-lactam cannot be removed and thus permanently renders the PBP incapable of its catalytic function. Ultimately, this results in death of bacterial cells from osmotic instability or autolysis.[4]
One of the main causes of resistance to β-lactam antibiotics is caused by β-lactamases. Chemically, β-lactamases and PBPs bind to β-lactam antibiotics in similar mechanisms, nucleophilic attack of the enzyme's catalytic serine on to the antibiotics carbonyl group. However, β-lactamases are then able to deactivate the antimicrobial activity of the β-lactams by cleaving the β-lactam bound in the [5] through a molecular process called deacylation, rendering it incapable of inhibiting the PBPs and ultimately, allowing cross-linking to occur for adequate cell wall formation.
History
Many medical textbooks recount Alexander Flemming’s serendipitous observation of the antibacterial action of Penicillium mold in 1928. While not the first to note the inhibitory effect, Flemming became the first to seriously push the scientific community to research the isolation of the active compound, which he named penicillin. Eventually these efforts paid off and in 1940, Sir Ernst Boris Chain published a method of isolating and purifying penicillin and tested its clinical effectiveness in mice. Later that year, he identified the first β-lactamase in Escherichia coli. While interesting, this discovery was not considered clinically relevant until β-lactamase enzymes were isolated from clinical samples of gram positive bacteria by Dr. William M. M. Kirby.
| |||||||||||
Disease
Placeholder
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ "Peptidoglycan cell wall." The University of Warwick. n.d. Web. 25 Jan 15
- ↑ Beta Lactam Antibiotics, 2011. Antimicrobial Resistance Learning Site. Michigan State University Department of Pharmacology. 16 Sept, 2014.
- ↑ Powers, Rachel, Hollister C. Swanson, Magdalena A. Taracila, Nicholas W. Florek, Chiara Romagnoli, Emilia Caselli, Fabio Prati, Robert A. Bonomo, and Bradley J. Wallar. Biochemical and Structural Analysis of Inhibitors Targeting the ADC-7 Cephalosporinase of Acinetobacter baumannii. Biochemistry, 2014, 53 (48), 7670-7679.