Sandbox myosinkinesin
From Proteopedia
| Line 4: | Line 4: | ||
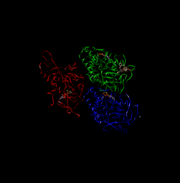
| - | [[Image:4UXR.png | thumb | This is 4UXR.]] | + | [[Image:4UXR.png | thumb | This is 4UXR, the head of kinesin.]] |
| Line 11: | Line 11: | ||
Kinesin is generally responsible for the transport of polypeptides and other large molecules throughout a single cell, such as moving the spindles during cell division. Kinesin can only move from the negative (-) end of microtubules to the positive (+) end. This means that kinesin only moves objects out towards the periphery of the cell and thus aids in the secretion of molecules or division of cells. | Kinesin is generally responsible for the transport of polypeptides and other large molecules throughout a single cell, such as moving the spindles during cell division. Kinesin can only move from the negative (-) end of microtubules to the positive (+) end. This means that kinesin only moves objects out towards the periphery of the cell and thus aids in the secretion of molecules or division of cells. | ||
== Disease == | == Disease == | ||
| - | Kinesin are involved during mitosis, failure of kinesin to work properly can lead to trisomy and other meiosis/mitosis related diseases. | + | Kinesin are involved during mitosis, failure of kinesin to work properly can lead to trisomy and other meiosis/mitosis related diseases. <ref>doi: 10.2210/rcsb_pdb/mom_2005_4</ref> |
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
| Line 26: | Line 25: | ||
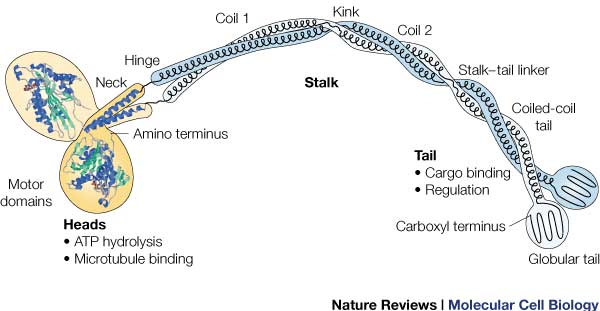
'''Head:''' | '''Head:''' | ||
| - | Most conserved domain amongst all kinesin, it consists mainly of alpha helixes. Its tertiary structure usually includes a large cleft from the actin binding site to the ATP binding pocket. The head has the ability to bind microtubule on one site and bind ATP at another. This section undergoes the most conformational change and is responsible for the force that causes kinesin to move along the cytoskeleton. In kinesin, binding of ATP appears to have allosteric control over the binding of kinesin to the tubules, by a twisting of a β- sheet core, showing allosteric control between the subdomains within the protein. | + | Most conserved domain amongst all kinesin, it consists mainly of alpha helixes. Its tertiary structure usually includes a large cleft from the actin binding site to the ATP binding pocket. The head has the ability to bind microtubule on one site and bind ATP at another. This section undergoes the most conformational change and is responsible for the force that causes kinesin to move along the cytoskeleton. In kinesin, binding of ATP appears to have allosteric control over the binding of kinesin to the tubules, by a twisting of a β- sheet core, showing allosteric control between the subdomains within the protein. <ref>DOI: 10.1039/c3cp53367k</ref> |
ATPase: It generates this force using an ATPase either coupled with the myosin/kinesin or inherent within the head, the inherent ATPase uses a P-loop, which is a phosphate- binding loop used in adenylate kinase, Ras, and others. Both also create a similar environment for the γ-phosphate, which uses a conserve motif of Ser-Ser-Arg. | ATPase: It generates this force using an ATPase either coupled with the myosin/kinesin or inherent within the head, the inherent ATPase uses a P-loop, which is a phosphate- binding loop used in adenylate kinase, Ras, and others. Both also create a similar environment for the γ-phosphate, which uses a conserve motif of Ser-Ser-Arg. | ||
Revision as of 17:50, 17 December 2015
Kinesin
| |||||||||||
References
Goodsell, David. "Kinesin." RCSB PDB-101. Protein Data Bank, n.d. Web. 07 Nov. 2015.
Krukau, Aliaksei, Volker Knecht, and Reinhard Lipowsky. "Allosteric Control of Kinesin's Motor Domain by Tubulin: A Molecular Dynamics Study." Phys. Chem. Chem. Phys. Physical Chemistry Chemical Physics 16.13 (2014): 6189. Royal Society of Chemistry. Web. 6 Nov. 2015.
Kull, F. Jon, Elena P. Sablin, Rebecca Lau, Robert J. Fletterick, and Ronald D. Vale. "Crystal Structure of the Kinesin Motor Domain Reveals a Structural Similarity to Myosin." Nature 380.6574 (1996): 550-55. The National Center for Biotechnology Information. Web. 6 Nov. 2015.
Lodish, Harvey F., Arnold Berk, Paul Matsudaira, Chris A. Kaiser, Monty Krieger, Matthew P. Scott, Stephen Lawrence Zipursky, and James E. Darnell. Molecular Cell Biology. New York, NY: W.H. Freemann, 2003. Print.
Paxton, Walter F., Nathan F. Bouxsein, Ian M. Henderson, Andrew Gomez, and George D. Bachand. "Dynamic Assembly of Polymer Nanotube Networks via Kinesin Powered Microtubule Filaments." Nanoscale 7.25 (2015): 10998-1004. Royal Society of Chemistry. Web. 6 Nov. 2015.
Rayment, I., W. Rypniewski, K. Schmidt-Base, R. Smith, D. Tomchick, M. Benning, D. Winkelmann, G. Wesenberg, and H. Holden. "Three-dimensional Structure of Myosin Subfragment-1: A Molecular Motor." Science 261.5117 (1993): 50-58. PubMed. Web. 6 Nov. 2015.
Rice, Sarah, Abel W. Lin, Daniel Safer, Cynthia L. Hart, Nariman Naber, Bridget O. Carragher, Shane M. Cain, Elena Pechatnikova, Elizabeth M. Wilson-Kubalek, Michael Whittaker, Edward Pate, Roger Cooke, Edwin W. Taylor, Ronald A. Milligan, and Ronald D. Vale. "A Structural Change in the Kinesin Motor Protein That Drives Motility." Nature 402.6763 (1999): 778-84. Nature. Web. 6 Nov. 2015.
"SDSU Biology 590 - Actin Myosin Crossbridge 3D Animation." SDSU Biology 590 - Actin Myosin Crossbridge 3D Animation. San Diego State University College of Sciences, 30 Sept. 97. Web. 07 Nov. 2015.
Song, Y.-H. "Structure of a Fast Kinesin: Implications for ATPase Mechanism and Interactions with Microtubules." The EMBO Journal 20.22 (2001): 6213-225. PubMed. Web. 6 Nov. 2015.
https://labs.cellbio.duke.edu/kinesin/KinesinStructure.html
- ↑ doi: https://dx.doi.org/10.2210/rcsb_pdb/mom_2005_4
- ↑ Krukau A, Knecht V, Lipowsky R. Allosteric control of kinesin's motor domain by tubulin: a molecular dynamics study. Phys Chem Chem Phys. 2014 Apr 7;16(13):6189-98. doi: 10.1039/c3cp53367k. PMID:24561904 doi:http://dx.doi.org/10.1039/c3cp53367k