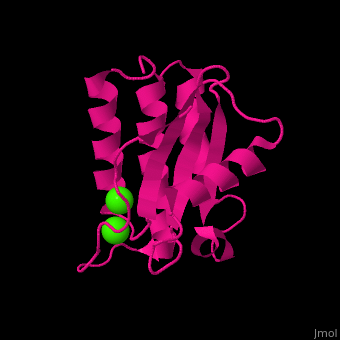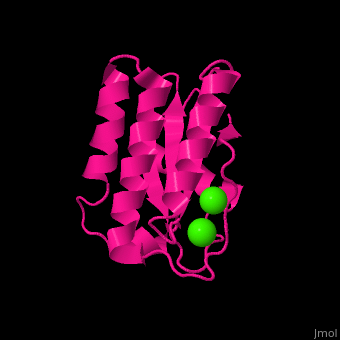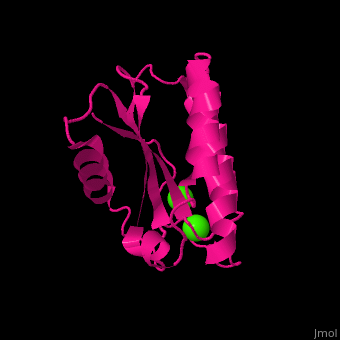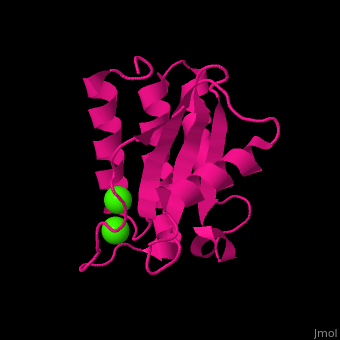BA42
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==BA42 Protein from ''Bizionia argentinensis''== | ==BA42 Protein from ''Bizionia argentinensis''== | ||
| - | <StructureSection load='4oa3' size='400' side='right' caption='Antarctic bacterium protein BA42 complex with Cl- ions (PDB code [[4oa3]])' scene=''> | + | <StructureSection load='4oa3' size='400' side='right' caption='Antarctic bacterium protein BA42 complex with Cl- ions (PDB code [[4oa3]])' scene='71/715464/Cv/1'> |
== Function == | == Function == | ||
| Line 7: | Line 7: | ||
Protein phosphatase from ''Bizionia argentinensis'' | Protein phosphatase from ''Bizionia argentinensis'' | ||
== Structural highlights == | == Structural highlights == | ||
| - | '''BA42''' belongs to the TPM protein family from Pfam. The TPM domain family is named after the three founding proteins TLP18.3, Psb32 and MOLO-1. TPM domains have a characteristic fold (αβαβαββαα or βαβαββαα) composed of α helices (3+3<ref>pmid 21908686</ref> or 2+3<ref>pmid 22198206</ref>) flanking four central β strands. The TPM fold has not been found in other protein domains to date. TPM was previously referred to as "DUF477" and "Repair_PSII". | + | '''BA42''' belongs to the TPM protein family from Pfam. The TPM domain family is named after the three founding proteins TLP18.3, Psb32 and MOLO-1. TPM domains have a characteristic fold <scene name='71/715464/Cv/2'>(αβαβαββαα or βαβαββαα)</scene> composed of α helices (3+3<ref>pmid 21908686</ref> or 2+3<ref>pmid 22198206</ref>) flanking four central β strands. The TPM fold has not been found in other protein domains to date. TPM was previously referred to as "DUF477" and "Repair_PSII". |
In plants, the TPM domain-containing proteins TLP18.3 and Psb32 that have been implicated in the photosystem II (PSII) repair cycle. It may be involved in the regulation of synthesis/degradation of the D1 protein of the PSII core and in the assembly of PSII monomers into dimers in the grana stacks.<ref>pmid 17576201</ref> | In plants, the TPM domain-containing proteins TLP18.3 and Psb32 that have been implicated in the photosystem II (PSII) repair cycle. It may be involved in the regulation of synthesis/degradation of the D1 protein of the PSII core and in the assembly of PSII monomers into dimers in the grana stacks.<ref>pmid 17576201</ref> | ||
Revision as of 11:09, 13 January 2016
BA42 Protein from Bizionia argentinensis
| |||||||||||
3D structure of BA42
2mpb - BaBA42 - Bizonia argentinesis - NMR
4oa3 - BaBA42
References
- ↑ Wu HY, Liu MS, Lin TP, Cheng YS. Structural and Functional Assays of AtTLP18.3 Identify Its Novel Acid Phosphatase Activity in Thylakoid Lumen. Plant Physiol. 2011 Sep 9. PMID:21908686 doi:10.1104/pp.111.184739
- ↑ Eletsky A, Acton TB, Xiao R, Everett JK, Montelione GT, Szyperski T. Solution NMR structures reveal a distinct architecture and provide first structures for protein domain family PF04536. J Struct Funct Genomics. 2012 Mar;13(1):9-14. doi: 10.1007/s10969-011-9122-2., Epub 2011 Dec 24. PMID:22198206 doi:http://dx.doi.org/10.1007/s10969-011-9122-2
- ↑ Sirpio S, Allahverdiyeva Y, Suorsa M, Paakkarinen V, Vainonen J, Battchikova N, Aro EM. TLP18.3, a novel thylakoid lumen protein regulating photosystem II repair cycle. Biochem J. 2007 Sep 15;406(3):415-25. PMID:17576201 doi:http://dx.doi.org/10.1042/BJ20070460
- ↑ Boulin T, Rapti G, Briseno-Roa L, Stigloher C, Richmond JE, Paoletti P, Bessereau JL. Positive modulation of a Cys-loop acetylcholine receptor by an auxiliary transmembrane subunit. Nat Neurosci. 2012 Oct;15(10):1374-81. doi: 10.1038/nn.3197. Epub 2012 Aug 26. PMID:22922783 doi:http://dx.doi.org/10.1038/nn.3197
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Martin Aran, Alexander Berchansky, Joel L. Sussman