We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Farnesyltransferase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
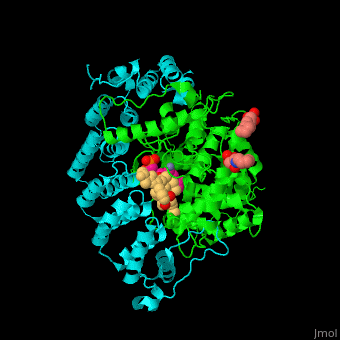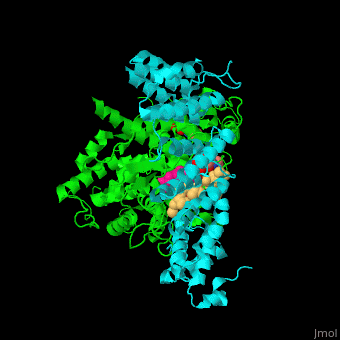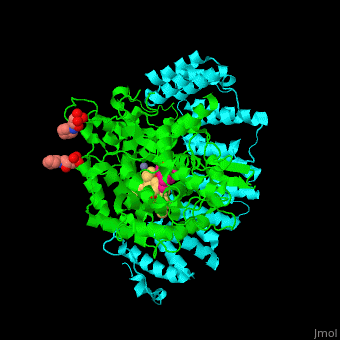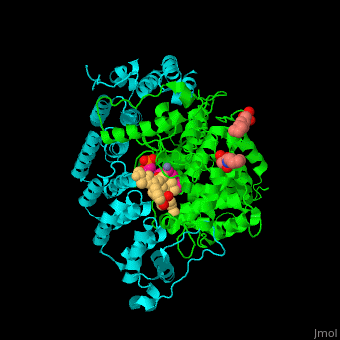
| - | <StructureSection load='3q75' size='350' side='right' scene='' caption=' Farnesyltransferase α subunit ( | + | <StructureSection load='3q75' size='350' side='right' scene='48/485616/Cv/1' caption=' Farnesyltransferase α subunit (cyan) and β subunit (green) complex with farnesyl diphosphate (FPP) analog, Zn+2 ion (grey), sulfonic acid derivative and CAAX peptide (pink) (PDB code [[3q75]]) '> |
| - | <!-- | ||
| - | Please use the "3D" button above this box to insert a Jmol applet (molecule) on this page. | ||
| - | Or use the four-green-boxes-button to insert scrollable text adjacent | ||
| - | to a Jmol applet. Check out the other buttons as well! | ||
| - | --> | ||
'''Farnesyltransferase''' (FTase) is part of the prenyltransferase group. FTase modifies proteins by adding farnesyl diphosphate (FPP) – an isoprenoid lipid – to a cysteine in a CAAX motif near the C terminal. This addition forms a thioether linkage, makes the protein more hydrophobic and associates it with the membrane. Farnesylated proteins – like those of the Ras family - are involved in cellular signaling<ref>PMID:15451670</ref> | '''Farnesyltransferase''' (FTase) is part of the prenyltransferase group. FTase modifies proteins by adding farnesyl diphosphate (FPP) – an isoprenoid lipid – to a cysteine in a CAAX motif near the C terminal. This addition forms a thioether linkage, makes the protein more hydrophobic and associates it with the membrane. Farnesylated proteins – like those of the Ras family - are involved in cellular signaling<ref>PMID:15451670</ref> | ||
. | . | ||
Revision as of 08:51, 25 January 2016
| |||||||||||
3D structures of farnesyltransferase
Updated on 25-January-2016
References
- ↑ Reid TS, Terry KL, Casey PJ, Beese LS. Crystallographic analysis of CaaX prenyltransferases complexed with substrates defines rules of protein substrate selectivity. J Mol Biol. 2004 Oct 15;343(2):417-33. PMID:15451670 doi:10.1016/j.jmb.2004.08.056
- ↑ Hast MA, Nichols CB, Armstrong SM, Kelly SM, Hellinga HW, Alspaugh JA, Beese LS. Structures of Cryptococcus neoformans Protein Farnesyltransferase Reveal Strategies for Developing Inhibitors That Target Fungal Pathogens. J Biol Chem. 2011 Oct 7;286(40):35149-62. Epub 2011 Aug 4. PMID:21816822 doi:10.1074/jbc.M111.250506