We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1125
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
==== Ca2+ ==== | ==== Ca2+ ==== | ||
[[Image:CA_pocket_interaction.gif | thumb|CA pocket interaction]] | [[Image:CA_pocket_interaction.gif | thumb|CA pocket interaction]] | ||
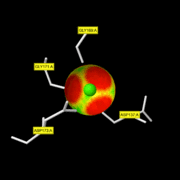
| - | + | The active site of the enzyme is composed by two Gly residues (169 and 171) next to two Asp residues (137 and 173). | |
| - | + | <font color='red'>maintain a Zn2+ atom coordinated with three water molecules. One of them is as well bound to the Glu residue thanks to a hydrogen bond. The second Zn2+ atom is not involved in the active site. At first, the Gly 206 residue of the substrate binds the active site thanks to the Zn2+ atom. When it binds it takes the place of unstable water molecules and establishes stabilizing interactions with the active site thanks to its C terminal part. Then, the Ala 182 residue of the enzyme makes a hydrogen bond with the NH group of the substrate: this allows the substrate to enter the cavity of the catalytic site. The rest of the protein is stabilized by 4 hydrogen bonds with the amino acid located in the cavity.</font> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==== Zn2+ ==== | ==== Zn2+ ==== | ||
[[Image:ZN pocket interaction.gif | thumb|ZN pocket interaction]] | [[Image:ZN pocket interaction.gif | thumb|ZN pocket interaction]] | ||
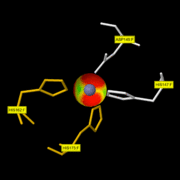
| - | + | The active site of the enzyme is composed by one Asp residue (149) next to three His residues (147, 162 and 175). | |
| + | <font color='red'>maintain a Zn2+ atom coordinated with three water molecules. One of them is as well bound to the Glu residue thanks to a hydrogen bond. The second Zn2+ atom is not involved in the active site. At first, the Gly 206 residue of the substrate binds the active site thanks to the Zn2+ atom. When it binds it takes the place of unstable water molecules and establishes stabilizing interactions with the active site thanks to its C terminal part. Then, the Ala 182 residue of the enzyme makes a hydrogen bond with the NH group of the substrate: this allows the substrate to enter the cavity of the catalytic site. The rest of the protein is stabilized by 4 hydrogen bonds with the amino acid located in the cavity.</font> | ||
Revision as of 20:25, 26 January 2016
MMP8
| |||||||||||
References
- ↑ "MMP8 matrix metallopeptidase 8 (neutrophil collagenase)"
- ↑ Stams T, Spurlino JC, Smith DL, Wahl RC, Ho TF, Qoronfleh MW, Banks TM, Rubin B. Structure of human neutrophil collagenase reveals large S1' specificity pocket. Nat Struct Biol. 1994 Feb;1(2):119-23. PMID:7656015