We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1125
From Proteopedia
(Difference between revisions)
| Line 43: | Line 43: | ||
== Function == | == Function == | ||
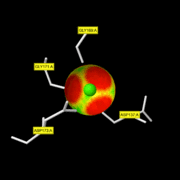
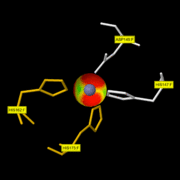
| - | Like all the MMPs, MMP8 is secreted as inactive proproteins and then activated after a cleavage by extracellular proteinases. Indeed, it can't be activated without removal of the <scene name='71/719866/Activation_peptide/ | + | Like all the MMPs, MMP8 is secreted as inactive proproteins and then activated after a cleavage by extracellular proteinases. Indeed, it can't be activated without removal of the <scene name='71/719866/Activation_peptide/2'>activation peptide</scene>. |
<font color='red'>cleave the helical collagen molecule at a single Gly-Ile/Leu bond in each alpha-chain of the molecule. The cleavage generates fragments that spontaneously lose their helical conformation, denature to gelatin, and become soluble. The gelatin is then susceptible to attack by gelatinases and other proteases</font><ref>[http://www.ebi.ac.uk/interpro/entry/IPR028709 "Neutrophil collagenase"]</ref> | <font color='red'>cleave the helical collagen molecule at a single Gly-Ile/Leu bond in each alpha-chain of the molecule. The cleavage generates fragments that spontaneously lose their helical conformation, denature to gelatin, and become soluble. The gelatin is then susceptible to attack by gelatinases and other proteases</font><ref>[http://www.ebi.ac.uk/interpro/entry/IPR028709 "Neutrophil collagenase"]</ref> | ||
<font color='red'>metalloendopeptidase activity = mechanism in which water acts as a nucleophile, one or two metal ions hold the water molecule in place, and charged amino acid side chains are ligands for the metal ions.</font><ref>[http://www.ebi.ac.uk/QuickGO/GTerm?id=GO:0004222#info=4 "Metalloendopeptidase activity"]</ref> | <font color='red'>metalloendopeptidase activity = mechanism in which water acts as a nucleophile, one or two metal ions hold the water molecule in place, and charged amino acid side chains are ligands for the metal ions.</font><ref>[http://www.ebi.ac.uk/QuickGO/GTerm?id=GO:0004222#info=4 "Metalloendopeptidase activity"]</ref> | ||
Revision as of 20:49, 27 January 2016
MMP8
Is enzyme : EC=3.4.24.34 => Hydrolase Called Neutrophil collagenase. zinc-dependent and calcium-dependent proteases that degrade most components of the extracellular matrix
| |||||||||||
References
- ↑ "MMP8 matrix metallopeptidase 8 (neutrophil collagenase)"
- ↑ Stams T, Spurlino JC, Smith DL, Wahl RC, Ho TF, Qoronfleh MW, Banks TM, Rubin B. Structure of human neutrophil collagenase reveals large S1' specificity pocket. Nat Struct Biol. 1994 Feb;1(2):119-23. PMID:7656015
- ↑ "Neutrophil collagenase"
- ↑ "Metalloendopeptidase activity"
RESSOURCE : Image:2oy4 mm1.pdb ( la structure du monomère )