Human BCL-2 (B-Cell Lymphoma 2), isoform 1 is an oncoprotein of 239 residues regulating cell death (apoptosis), notably acting as an anti-apoptotic. It is encoded by the BCL2 gene located on the 18th chromosome (63.12-63.32 Mb). There are 2 isoforms of this protein (𝛼 and 𝛽), produced by alternative splicing, and which differ by 2 aminoacids (residues 96 (A→T) and 110 (R→G))(ref). Alteration of this protein is a caused of many cancers, and is also likely to be involved in schyzophrenia and autoimmunity.
Structure
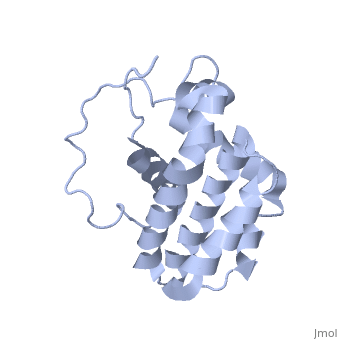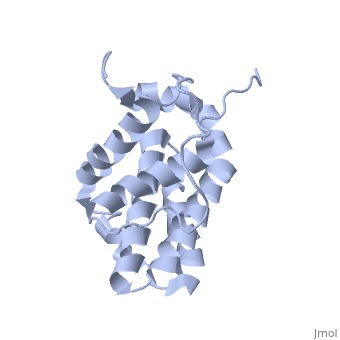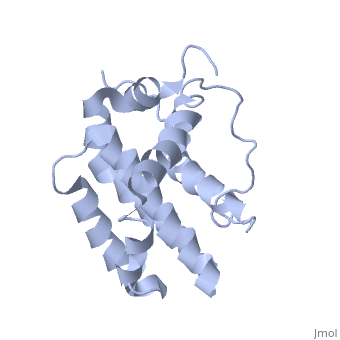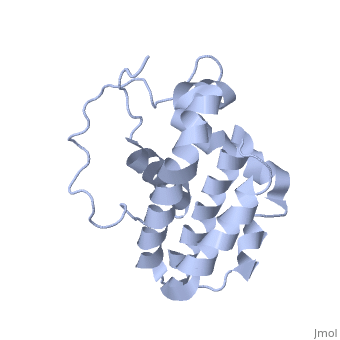
Human BCL-2, isoform 1 is a 26kDa protein of 239 residues which is negatively charged at pH 7. The linear structure highlights 5 domains: (10-30),
(93-107), (136-155), (187-202) and a
transmembrane domain (218-239) (due to its poor behavior in solution, it has been replaced by a segment of Bcl-xl in the presented 3D structure). It organizes as eight alpha-helices: from 11 to 25 (1) , from 93 to 107 (2), from 109 to 118 (3), from 126 to 137 (4), from 144-163 (5), from 169 to 184 (6), from 186 to 191 (7) and from 194 to 202(8). Helices 5 and 6 are mostly hydrophobic and they are surrounded by four other helices characterized by their amphipathic properties. There are also 3 turns (32-34, 123-125, 138-140). The is a 3(10) helix, whereas BCL-XL 3rd helix is a normal alpha-helix.
The transmembrane domain of Bcl-2 is made of 21 aminoacids and is located at the carboxy-terminal tail of the protein. It allows the docking of Bcl-2 in the mitochondrial outer membrane where the protein interacts with other effectors.[1]
The tertiary structure of Bcl-2 shows that this protein contains a hydrophobic groove on its surface that allows dimerization with other members of the Bcl-2 family. This region needs to be highly conserved to keep the ability of interacting with proapoptotic protein of the family, in fact, it has been shown that a mutation in this structure leads to the silencing of the dimerization thus may inhibit the activity of Bcl-2.
The isoform 1 and 2 differs from two amino acid in the hydrophobic groove but this difference doesn’t induce any change in the conformation of this protein.
However as expected, it affect the affinity with Bad and Bak proteins (from the Bcl-2 family). Indeed Bcl-2 isoform 1 shows to have a weaker affinity for Bad and Bak compared to isoform 2.[2]
Function
IP3R inhibition
Bcl-2 localized at the endoplasmic reticulum (ER) membranes participates in the control of Ca2+ content and release. The inositol 1,4,5-trisphosphate receptor (IP3R) is the primary Ca2+ release channel localized in the ER. Its pro-apoptotic activity can be directly inhibited by the Bcl-2, homology domain 4 (BH4) being essential and sufficient for this effect. comprises 20 amino acids (10-30) organized in alpha-helical structure which is required to inhibit IP3R. Residues K17, H20, Y21 and R26 participate in the inhibition of IP3R because they are very accessible and proximal in the secondary structure. [3]
Regulation of the mitochondrial pathway of apoptosis
BH3-only proteins which belong to the Bcl-2 family activate pro-apoptotic proteins such as Bcl-2-associated X protein (Bax) or Bcl-2 antagonist/killer-1 (Bak) at the mitochondrion. When Bax or Bak are activated, they homo-oligomerize and form the pores in the outer mitochondrial membrane which are necessary for the pro-apoptotic molecules (including second mitochondria-derived activator of caspase and cytochrome c) to escape. Then cytochrome c leads to the activation of caspases which are actually proteases that degrade the key proteins of the cell.
On the other hand, Bcl-2 may prevent the activation and homo-oligomerization of Bax and Bak thus blocking the cell death. This is achieved by sequestering BH3-only proteins or activated and monomeric Bax and Bak. and are essential for Bcl-2/Bax heterodimer formation. The conservation of each amino acid seems to be very important to this interaction. [4][5]
Therefore, the neutralization of Bcl-2 is required for eficient cell-death. BH3 proteins Bad, Bim and Puma bind Bcl-2 and disable its anti-apoptotic activity. BH3 peptides occupy the hydrophobic pocket of Bcl-2 and the sequestered pro-apoptotic proteins are released. This hydrophobic pocket is formed by F97 and Y101. [6] [7]
Bcl-2 localized on external mitochondrial membrane can also inhibit the release of cytochrome c from mitochondria. [8] [9]
Regulation of proinflammatory caspase-1 activation
NALP1 is a member of a NLR-family proteins. Its function is to activate the members of the proinflammatory caspase family which participate in cytokines activation pathway (especially caspase-1). The Bcl-2 loop regions between the BH3 and BH4 bind NALP1. This interaction is exclusively reserved to two members of Bcl-2 family: Bcl-XL and Bcl-2 itself because this interacting region is highly variable in Bcl-2 family. By binding to NALP1, Bcl-2 inhibits the inflammatory caspase activation. Hence, it protects cell from the stress.
The posttranslational modifications found on the loops between BH3 and BH4 modify the anti-apoptotic activity of Bcl-2. Hence, the Bcl-2 binding to NALP1 can be affected by these modifications. [10]
Diseases
Cancer
BCL-2 is involved in many cancers such as breast, melanoma, prostate, chronic lymphocytic leukemia and lung cancers. In fact, its overexpression combined with an overexpression of c-Myc (another oncoprotein) produce aggressive B-lymphocytes. [11]. It has also be shown that BCL-2 overpexpression supresses DNA repair by enhancing Myc transcriptional activity.
[12] In fact, BCL-2 is encoded by the BCL-2 gene (0,20 Mb) located on the 18th chromosome. Nevertheless, translocation between chromosome 14 and 18 juxtaposes the BCL-2 gene and the immunoglobulin locus. This brings the BCL-2 gene under the regulation of the immunoglobulin heavy-chain enhancer, diregulating BCL-2 expression level (involved in non-Hodgkin's lymphomas). [13]
Other mechanisms appear to regulate and enhance the level of expression of BCL-2 : loss of endogenous microRNAs which normally repress BCL-2 expression, and also hypomethylation.[14]
BCL-2 BH4 domain mediates the interaction with MBII domain of Myc. This interaction enhance c-Myc half-life, resulting in an enhancing of its total activity. c-Myc is a well known oncoprotein (transcription factor) involved in many cancers as it regulates a lot of genes of the cell cycle.
Structural highlights