We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1126
From Proteopedia
(Difference between revisions)
| Line 56: | Line 56: | ||
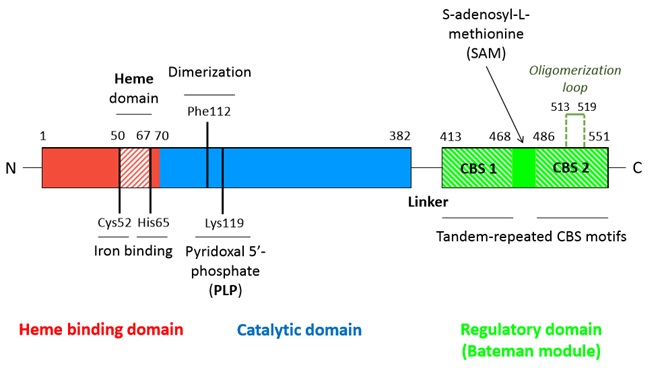
*Activation by SAM : | *Activation by SAM : | ||
| - | S-adenosyl-methionine seems to bind in a region located between the CBS1 and CBS2 domains of the Bateman module since it is solvent-exposed and has no hefty residues. Moreover, this region shapes a hydrophobic cage able to host the adenine ring | + | S-adenosyl-methionine seems to bind in a region located between the CBS1 and CBS2 domains of the Bateman module since it is solvent-exposed and has no hefty residues. Moreover, this region shapes a hydrophobic cage able to host the adenine ring. Moreover threonine (T535) and aspartate (D538) help stabilizing the ribose through hydrogen bounds and polar interactions. |
S-adenosyl-methionine (SAM) is the allosteric regulator factor of the CBS. Its binding to the CBS2 domain of the Bateman module destabilizes the interactions which sustain the tetramer structure and thus triggers the dissocciation of the tetrameric structure into two dimers. | S-adenosyl-methionine (SAM) is the allosteric regulator factor of the CBS. Its binding to the CBS2 domain of the Bateman module destabilizes the interactions which sustain the tetramer structure and thus triggers the dissocciation of the tetrameric structure into two dimers. | ||
Revision as of 12:55, 29 January 2016
| This Sandbox is Reserved from 15/12/2015, through 15/06/2016 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1120 through Sandbox Reserved 1159. |
To get started:
More help: Help:Editing |
Human cystathionine β-synthase (hCBS)
| |||||||||||