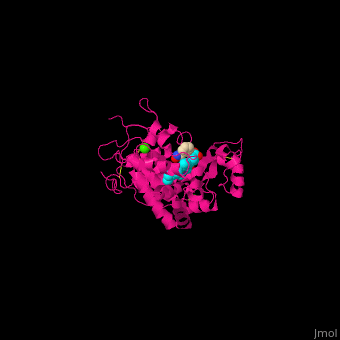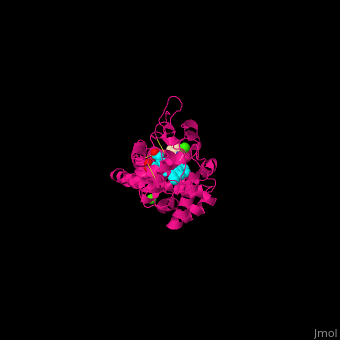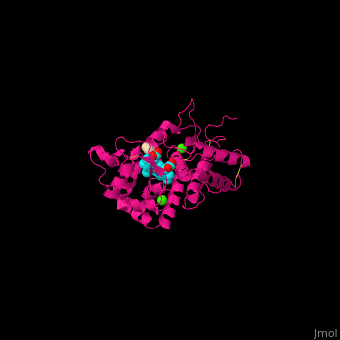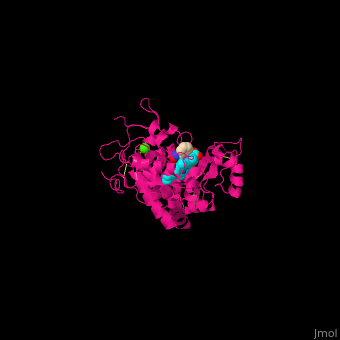Horseradish peroxidase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='2atj' size='350' side='right' caption='Horseradish peroxidase containing heme group complex with benzhydroxamic acid and Ca+2 ion (green) (PDB entry [[2atj]])' scene=''> | + | <StructureSection load='2atj' size='350' side='right' caption='Horseradish peroxidase containing heme group complex with benzhydroxamic acid and Ca+2 ion (green) (PDB entry [[2atj]])' scene='48/486455/Cv/1'> |
== Function == | == Function == | ||
'''Horseradish peroxidase''' (HRP) catalyzes the oxidation of luminol to 3-aminophthalate using hydrogen peroxide. The reaction is accompanied by emission of light. In the presence of chemicals, particularly – p-iodophenol, the light emission is enhanced 1000-fold. Upon addition of the proper substrate, the HRP complex emits colored light. HRP contains a heme group<ref>PMID:14751298</ref>. | '''Horseradish peroxidase''' (HRP) catalyzes the oxidation of luminol to 3-aminophthalate using hydrogen peroxide. The reaction is accompanied by emission of light. In the presence of chemicals, particularly – p-iodophenol, the light emission is enhanced 1000-fold. Upon addition of the proper substrate, the HRP complex emits colored light. HRP contains a heme group<ref>PMID:14751298</ref>. | ||
Revision as of 12:29, 3 April 2016
| |||||||||||
3D structures of horseradish peroxidase
Updated on 03-April-2016
References
- ↑ Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004 Feb;65(3):249-59. PMID:14751298
- ↑ Henriksen A, Schuller DJ, Meno K, Welinder KG, Smith AT, Gajhede M. Structural interactions between horseradish peroxidase C and the substrate benzhydroxamic acid determined by X-ray crystallography. Biochemistry. 1998 Jun 2;37(22):8054-60. PMID:9609699 doi:10.1021/bi980234j