We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Daniel Schemenauer/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Introduction == | == Introduction == | ||
G-coupled protein receptors [https://en.wikipedia.org/wiki/G_protein–coupled_receptor GPCR] are trans-membrane proteins that are integral to cell signaling. The human genome encodes for approximately 750 GPCRS, 350 of which are known to respond to extracellular ligands.<ref name="GPCRRep">PMID: 12679517 </ref>. GPCRs are divided into four major classes based on sequence similarity and transduction mechanism; Class A,B,C, and F.<ref name="MSGPCR">PMID:23407534</ref>. Metabotropic Glutamate Receptor 5 (mGlu<sub>5</sub>) is a class C GPCR that is involved in the G<sub>q</sub> pathway <ref name="CCGPCR">PMID:12782243</ref>. mGlu<sub>5</sub>is highly expressed in neuronal and glial cells in the central nervous system, where glutamate serves as the major neurotransmitter. When glutamate binds to the extracellular domain of mGlu<sub>5</sub>, consisting of the Venus Fly Trap <ref name="Primary">PMID: 25042998 </ref>, a conformational change through the tram-membrane domains activates the coupled G-protein. This G-protein disassociates and the alpha subunit activates [https://en.wikipedia.org/wiki/Phospholipase_C Phospholipase C] which has the final outcome of increased neuronal activity<ref name="MSGPCR">PMID:23407534</ref>. | G-coupled protein receptors [https://en.wikipedia.org/wiki/G_protein–coupled_receptor GPCR] are trans-membrane proteins that are integral to cell signaling. The human genome encodes for approximately 750 GPCRS, 350 of which are known to respond to extracellular ligands.<ref name="GPCRRep">PMID: 12679517 </ref>. GPCRs are divided into four major classes based on sequence similarity and transduction mechanism; Class A,B,C, and F.<ref name="MSGPCR">PMID:23407534</ref>. Metabotropic Glutamate Receptor 5 (mGlu<sub>5</sub>) is a class C GPCR that is involved in the G<sub>q</sub> pathway <ref name="CCGPCR">PMID:12782243</ref>. mGlu<sub>5</sub>is highly expressed in neuronal and glial cells in the central nervous system, where glutamate serves as the major neurotransmitter. When glutamate binds to the extracellular domain of mGlu<sub>5</sub>, consisting of the Venus Fly Trap <ref name="Primary">PMID: 25042998 </ref>, a conformational change through the tram-membrane domains activates the coupled G-protein. This G-protein disassociates and the alpha subunit activates [https://en.wikipedia.org/wiki/Phospholipase_C Phospholipase C] which has the final outcome of increased neuronal activity<ref name="MSGPCR">PMID:23407534</ref>. | ||
| - | |||
== Structure == | == Structure == | ||
| Line 16: | Line 15: | ||
===Role in Diseases=== | ===Role in Diseases=== | ||
mGlu<sub>5</sub> is located mainly post-synaptically and is in high abundance in the [https://en.wikipedia.org/wiki/Nucleus_accumbens nucleus accumbens], [https://en.wikipedia.org/wiki/Caudate_nucleus caudate nucleus], [https://en.wikipedia.org/wiki/Striatum striatum], [https://en.wikipedia.org/wiki/Hippocampus hippocampus] and [https://en.wikipedia.org/wiki/Cerebellum cerebellar cortex] <ref name="Local">PMID: 8295733 </ref>.These areas of the brain are highly involved in cognition, motivation and emotion, essential neural functions for everyday life. Diseases and other mental deficiencies arise from either an over activation of the GPCR, which over activates its coupled signaling pathway, or from under activation of both. Negative allosteric modulators (NAMs) work to decrease protein activity and are being studied as treatments for [https://en.wikipedia.org/wiki/Fragile_X_syndrome fragile X-syndrome], depression, anxiety and [https://en.wikipedia.org/wiki/Dyskinesia dyskinesia]. Conversely Positive allosteric modulators work to increase protein activity and are being studied for the treatment of schizophrenia and cognitive disorders<ref name="Diseases">PMID: 24237242</ref>. | mGlu<sub>5</sub> is located mainly post-synaptically and is in high abundance in the [https://en.wikipedia.org/wiki/Nucleus_accumbens nucleus accumbens], [https://en.wikipedia.org/wiki/Caudate_nucleus caudate nucleus], [https://en.wikipedia.org/wiki/Striatum striatum], [https://en.wikipedia.org/wiki/Hippocampus hippocampus] and [https://en.wikipedia.org/wiki/Cerebellum cerebellar cortex] <ref name="Local">PMID: 8295733 </ref>.These areas of the brain are highly involved in cognition, motivation and emotion, essential neural functions for everyday life. Diseases and other mental deficiencies arise from either an over activation of the GPCR, which over activates its coupled signaling pathway, or from under activation of both. Negative allosteric modulators (NAMs) work to decrease protein activity and are being studied as treatments for [https://en.wikipedia.org/wiki/Fragile_X_syndrome fragile X-syndrome], depression, anxiety and [https://en.wikipedia.org/wiki/Dyskinesia dyskinesia]. Conversely Positive allosteric modulators work to increase protein activity and are being studied for the treatment of schizophrenia and cognitive disorders<ref name="Diseases">PMID: 24237242</ref>. | ||
| - | |||
===Interactions with a Negative Allosteric Modulator=== | ===Interactions with a Negative Allosteric Modulator=== | ||
| Line 23: | Line 21: | ||
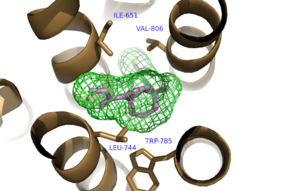
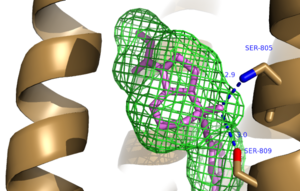
The bicyclic ring system of the drug is surrounded by a pocket of mainly hydrophobic residues including Val 806, Met 802, Phe 788, Trp 785, Leu 744, Ile 651, Pro 655 and Asn 747<ref name="Primary">PMID: 25042998 </ref> (Figure 1). The carbamate tail of mavoglurant forms a hydrogen bond through its carbonyl oxygen to the amide side-chain of Asparagine 747 of TM4 (Figure 2). A hydroxyl group similarly forms hydrogen bonds to the protein, specifically to two serine residues (S805 and S809) of TM7 which form a hydrogen bonding network to other residues through their main chain atoms and a coordinated water molecule (omitted for clarity) (Figure 3). The interactions between Mavoglurant andmGlu<sub>5</sub> involved TM helices that were not previously stabilized by any strong interactions, introducing a new level of stability that favors the inactive conformation of the protein and hence decrease activity<ref name="Primary">PMID: 25042998 </ref>. | The bicyclic ring system of the drug is surrounded by a pocket of mainly hydrophobic residues including Val 806, Met 802, Phe 788, Trp 785, Leu 744, Ile 651, Pro 655 and Asn 747<ref name="Primary">PMID: 25042998 </ref> (Figure 1). The carbamate tail of mavoglurant forms a hydrogen bond through its carbonyl oxygen to the amide side-chain of Asparagine 747 of TM4 (Figure 2). A hydroxyl group similarly forms hydrogen bonds to the protein, specifically to two serine residues (S805 and S809) of TM7 which form a hydrogen bonding network to other residues through their main chain atoms and a coordinated water molecule (omitted for clarity) (Figure 3). The interactions between Mavoglurant andmGlu<sub>5</sub> involved TM helices that were not previously stabilized by any strong interactions, introducing a new level of stability that favors the inactive conformation of the protein and hence decrease activity<ref name="Primary">PMID: 25042998 </ref>. | ||
[[Image:Mav_Hydrophobic_pocket.png |300 px|left|thumb|Figure 1.Hydrophobic Pocket Surrounding Mavoglurant]] | [[Image:Mav_Hydrophobic_pocket.png |300 px|left|thumb|Figure 1.Hydrophobic Pocket Surrounding Mavoglurant]] | ||
| - | [[Image:Mav_HB_1.1.png|300 px| | + | [[Image:Mav_HB_1.1.png|300 px|right|thumb|Figure 2.Hydrogen Bonding interactions between protein and Mavoglurant]] |
[[Image:Mav_HB_2.png|300 px|left|thumb|Figure 3. Further Hydrogen Bonding between protein and Mavoglurant]] | [[Image:Mav_HB_2.png|300 px|left|thumb|Figure 3. Further Hydrogen Bonding between protein and Mavoglurant]] | ||
| - | + | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 18:56, 28 March 2016
| |||||||||||
References
- ↑ Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, Bergmann JE, Gaitanaris GA. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4903-8. Epub 2003 Apr 4. PMID:12679517 doi:http://dx.doi.org/10.1073/pnas.0230374100
- ↑ 2.0 2.1 Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013 Feb 14;494(7436):185-94. doi: 10.1038/nature11896. PMID:23407534 doi:http://dx.doi.org/10.1038/nature11896
- ↑ Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003 Jun;98(3):325-54. PMID:12782243
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Dore AS, Okrasa K, Patel JC, Serrano-Vega M, Bennett K, Cooke RM, Errey JC, Jazayeri A, Khan S, Tehan B, Weir M, Wiggin GR, Marshall FH. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014 Jul 31;511(7511):557-62. doi: 10.1038/nature13396. Epub 2014 Jul 6. PMID:25042998 doi:http://dx.doi.org/10.1038/nature13396
- ↑ Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993 Nov 26;163(1):53-7. PMID:8295733
- ↑ Li G, Jorgensen M, Campbell BM. Metabotropic glutamate receptor 5-negative allosteric modulators for the treatment of psychiatric and neurological disorders (2009-July 2013). Pharm Pat Anal. 2013 Nov;2(6):767-802. doi: 10.4155/ppa.13.58. PMID:24237242 doi:http://dx.doi.org/10.4155/ppa.13.58