We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1160
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
== Human metabotropic glutamate receptor 5 transmembrane domain == | == Human metabotropic glutamate receptor 5 transmembrane domain == | ||
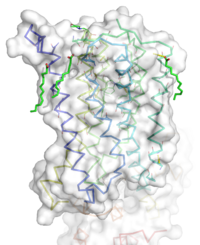
<StructureSection load='4oo9' size='350' frame='true' side='right' caption='Human metabotropic glutamate receptor 5 transmembrane domain' <'scene='(Ionic_lock)'> | <StructureSection load='4oo9' size='350' frame='true' side='right' caption='Human metabotropic glutamate receptor 5 transmembrane domain' <'scene='(Ionic_lock)'> | ||
| - | Receiving and responding to extracellular | + | Receiving and responding to extracellular messages is critical to the proper function of the nervous system. Glutamate is the major excitory neurotransmitter of the CNS, and metabotropic glutamate receptor 5 will play a major role in glutamate signaling. Metabotropic glutamate receptor 5 transmembrane domain is a homodimeric GPCR that resides in the cellular membrane. This domain is a member of the Class C GPCR family and can further be categorized into the Group I subgroup. The transmembrane domain will signal through a Gq/11 pathway. mGlu5 will bind glutamate to the extracellular Venus flytrap domain and the signal will be transduced across the membrane to a heterotrimeric G protein, which will ultimately lead to calcium release and activation of PKC. This will elicit a excitory post-synaptic repose and modulate long term potentiation. Human metabotropic glutamate receptor 5 is found throughout the central nervous system. Areas containing high concentrations of this protein are often involved involved in emotions and higher cognition. The localization of mGlu5 in the CNS and the presence of multiple domains makes mGlu5 a possible target for treating schizophrenia, Fragile X, depression, anxiety,and Alzheimer's disease. |
== Discovery == | == Discovery == | ||
| - | The mGlu | + | The mGlu family of receptors was the first of the Class C GPCR to be extensively studied. The first regions of the protein crystallized and studied were the Venus fly trap domain and the cystiene-rich domain on the extracellular region of the receptor. The hydrophobic nature and flexibility of the transmembrane domain made it difficult to crystalize. Recently, the human metabotropic glutamate receptor 5 transmembrane domain was crystallized and a structure elucidated. There were several modifications that had to be made. |
== Structure== | == Structure== | ||
[[Image:STR.png|200 px|left|thumb|Figure Legend]] | [[Image:STR.png|200 px|left|thumb|Figure Legend]] | ||
| + | === Overview === | ||
| + | The mGlu5 TMD contains 7 alpha helices that span the membrane. The protein was crystallized with Oleic acid and MES. On the superior portion of the protein there are several critical extracellular loops.The binding pocket can be found near the middle of the protein.Inserted into the biding pocket is the negative allosteric modulator Mavoglurant. It is important to note that the TMD as illustrated is in an inactive conformation. On the intracellular portion of the protein there exist several ionic locks whose positions will determine the activity of the protein. | ||
=== Extracellular Domain === | === Extracellular Domain === | ||
=== Binding Pocket === | === Binding Pocket === | ||
Revision as of 01:16, 29 March 2016
Human metabotropic glutamate receptor 5 transmembrane domain
| |||||||||||