We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1167
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Background == | == Background == | ||
[[Image:7tm_labeled_with_membrane.png|200 px|left|thumb|The 7tm spans the cell membrane]]The human glucagon receptor (GCGR) is one of 15 secretin-like, or Class B, G-protein-coupled receptors (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain (shown in blue) and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (shown in magenta). As its name suggests, the 7tm is made up of alpha helices that pass through the membrane seven times. The extracellular domain has an α-β-β structure that consists of two antiparallel β-sheets and a N-terminal α-helix<ref>PMID:26227798</ref>. | [[Image:7tm_labeled_with_membrane.png|200 px|left|thumb|The 7tm spans the cell membrane]]The human glucagon receptor (GCGR) is one of 15 secretin-like, or Class B, G-protein-coupled receptors (GPCRs). Like other GPCRs, it has a <scene name='72/721538/7tm_labeled_helicies/3'>7 trans-membrane </scene> helical domain (shown in blue) and a globular N-terminus <scene name='72/721538/Ecd/2'>extracellular domain</scene> (shown in magenta). As its name suggests, the 7tm is made up of alpha helices that pass through the membrane seven times. The extracellular domain has an α-β-β structure that consists of two antiparallel β-sheets and a N-terminal α-helix<ref>PMID:26227798</ref>. | ||
| - | |||
== Function == | == Function == | ||
The Glucagon Receptor plays an important role in glucose homeostasis. During times of fasting (or low blood sugar) the pancreas dispatches glucagon to activate the GCGR in the liver. The [http://www.nature.com/nature/journal/v499/n7459/fig_tab/nature12393_F5.html binding of glucagon] stimulates gluconeogenesis, through adenylate cyclase that initiates protein kinase A (PKA) activity<ref>PMID:25674162</ref>. This pathway synthesizes glucose, elevating blood sugar levels. | The Glucagon Receptor plays an important role in glucose homeostasis. During times of fasting (or low blood sugar) the pancreas dispatches glucagon to activate the GCGR in the liver. The [http://www.nature.com/nature/journal/v499/n7459/fig_tab/nature12393_F5.html binding of glucagon] stimulates gluconeogenesis, through adenylate cyclase that initiates protein kinase A (PKA) activity<ref>PMID:25674162</ref>. This pathway synthesizes glucose, elevating blood sugar levels. | ||
| - | |||
== Structure == | == Structure == | ||
| Line 26: | Line 24: | ||
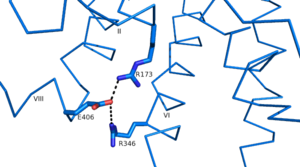
As a part of the interface stabilization between helices VI, V, and III, a Class B specific [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond] occurs between [https://en.wikipedia.org/wiki/Asparagine N] 318 of Helix V and [https://en.wikipedia.org/wiki/Leucine L] 242 of Helix III. | As a part of the interface stabilization between helices VI, V, and III, a Class B specific [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bond] occurs between [https://en.wikipedia.org/wiki/Asparagine N] 318 of Helix V and [https://en.wikipedia.org/wiki/Leucine L] 242 of Helix III. | ||
| - | |||
=== GCGR-Specific Traits === | === GCGR-Specific Traits === | ||
| Line 43: | Line 40: | ||
An important interface stabilization interaction between helices I and VII occurs between [https://en.wikipedia.org/wiki/Serine Ser] 152 of Helix I and Ser 390 of Helix VII. Due to their close proximity to one another, they form an important <scene name='72/721537/Ser-ser_hydrogen_bond/2'>hydrogen bond</scene> which stabilizes the structure of GCGR. | An important interface stabilization interaction between helices I and VII occurs between [https://en.wikipedia.org/wiki/Serine Ser] 152 of Helix I and Ser 390 of Helix VII. Due to their close proximity to one another, they form an important <scene name='72/721537/Ser-ser_hydrogen_bond/2'>hydrogen bond</scene> which stabilizes the structure of GCGR. | ||
| - | |||
== Glucagon Binding == | == Glucagon Binding == | ||
Revision as of 03:48, 30 March 2016
| This Sandbox is Reserved from Jan 11 through August 12, 2016 for use in the course CH462 Central Metabolism taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1160 through Sandbox Reserved 1184. |
To get started:
More help: Help:Editing |
Class B Human Glucagon G-Protein Coupled Receptor
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, Pascal BD, Wu B, Potter CS, Zhou H, Griffin PR, Carragher B, Yang H, Wang MW, Stevens RC, Jiang H. Conformational states of the full-length glucagon receptor. Nat Commun. 2015 Jul 31;6:7859. doi: 10.1038/ncomms8859. PMID:26227798 doi:http://dx.doi.org/10.1038/ncomms8859
- ↑ Lotfy M, Kalasz H, Szalai G, Singh J, Adeghate E. Recent Progress in the Use of Glucagon and Glucagon Receptor Antago-nists in the Treatment of Diabetes Mellitus. Open Med Chem J. 2014 Dec 31;8:28-35. doi: 10.2174/1874104501408010028., eCollection 2014. PMID:25674162 doi:http://dx.doi.org/10.2174/1874104501408010028