User:Dean Williams/Sandbox 1180
From Proteopedia
| Line 1: | Line 1: | ||
| - | ==Structure of Class B Human Glucagon G-Protein Coupled Receptors== | + | ==Structure of Class B Human Glucagon G-Protein Coupled Receptors (GCGRs)== |
G protein coupled receptors (GPCRs) are recognized as the largest known class of integral membrane proteins and are divided into five families; the rhodopsin family (class A), the secretin family (class B), the adhesion family, the glutamate family (class C), and the frizzled/taste family (class F). Roughly 5% of the human genome encodes g-protein-coupled receptors which are responsible for the transduction of endogenous signals and the instigation of cellular response. The variants all contain a similar seven α-helical transmembrane domain (TMD) that, once bound to its peptide ligand, undergoes conformational change and tranduces a signal to coupled, heterotrimeric G proteins which initiate intracellular signal pathways and generate physiological and pathological processes. <ref>DOI 10.1371/journal.pcbi.0020013</ref> | G protein coupled receptors (GPCRs) are recognized as the largest known class of integral membrane proteins and are divided into five families; the rhodopsin family (class A), the secretin family (class B), the adhesion family, the glutamate family (class C), and the frizzled/taste family (class F). Roughly 5% of the human genome encodes g-protein-coupled receptors which are responsible for the transduction of endogenous signals and the instigation of cellular response. The variants all contain a similar seven α-helical transmembrane domain (TMD) that, once bound to its peptide ligand, undergoes conformational change and tranduces a signal to coupled, heterotrimeric G proteins which initiate intracellular signal pathways and generate physiological and pathological processes. <ref>DOI 10.1371/journal.pcbi.0020013</ref> | ||
| Line 5: | Line 5: | ||
Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases concentration of cAMP, inositol phosphate, and calcium levels in cyto. <ref>DOI 10.1111/bph.12689</ref> These signals are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest to companies developing novel molecules. <ref>DOI 10.1016/j.tips.2013.11.001</ref> Structurally based approaches to the development of small-molecule agonists and antagonists have been hampered by the lack of accurate Class B TMD visualizations until recent crystal structures of corticoptropin-releasing factor receptor 1 and human glucagon were realized. <ref>DOI 10.1038/nature12357</ref> <ref>DOI 10.1038/nature12393</ref> | Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases concentration of cAMP, inositol phosphate, and calcium levels in cyto. <ref>DOI 10.1111/bph.12689</ref> These signals are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest to companies developing novel molecules. <ref>DOI 10.1016/j.tips.2013.11.001</ref> Structurally based approaches to the development of small-molecule agonists and antagonists have been hampered by the lack of accurate Class B TMD visualizations until recent crystal structures of corticoptropin-releasing factor receptor 1 and human glucagon were realized. <ref>DOI 10.1038/nature12357</ref> <ref>DOI 10.1038/nature12393</ref> | ||
| - | The glucagon class B GPCR is | + | The glucagon class B GPCR (GCGR) is involved in glucose homeostasis through the binding of the signal peptide glucagon. |
| Line 11: | Line 11: | ||
<StructureSection load='4L6R' size='340' side='right' caption='7TM Helical Structure of 4L6R GPCR' scene='72/727084/Scene_1/2'> | <StructureSection load='4L6R' size='340' side='right' caption='7TM Helical Structure of 4L6R GPCR' scene='72/727084/Scene_1/2'> | ||
This is a default text for your page '''Allie Paton/Sandbox 1181'''. Click above on '''http://sbkb.org/fs/glucagon-receptor''' to modify. Be careful with the < and > signs. | This is a default text for your page '''Allie Paton/Sandbox 1181'''. Click above on '''http://sbkb.org/fs/glucagon-receptor''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: | + | You may include any references to papers as in: using DOI # <ref>DOI 10.1038/nature12393</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. |
<scene name='72/727084/Scene_1/2'>Nucleotide 200-205</scene> | <scene name='72/727084/Scene_1/2'>Nucleotide 200-205</scene> | ||
| Line 19: | Line 19: | ||
Glucagon is released from pancreatic α-cells when blood glucose levels fall after a period of fasting or several hours following intake of dietary carbohydrates. Once the peptide hormone is released, it binds to GCGRs initiating signaling to heterotrimeric G-proteins containing Gαs. <ref>DOI 10.1038/nrd2782</ref> Additionally, GCGR can regulate additional signal pathways including G-proteins of the Gαi family through the adoption of differing receptor conformations. <ref>DOI 10.3109/10799890903295150</ref> | Glucagon is released from pancreatic α-cells when blood glucose levels fall after a period of fasting or several hours following intake of dietary carbohydrates. Once the peptide hormone is released, it binds to GCGRs initiating signaling to heterotrimeric G-proteins containing Gαs. <ref>DOI 10.1038/nrd2782</ref> Additionally, GCGR can regulate additional signal pathways including G-proteins of the Gαi family through the adoption of differing receptor conformations. <ref>DOI 10.3109/10799890903295150</ref> | ||
| - | Activates adenylate cyclase | ||
| - | |||
| - | Upregulates intracellular cAMP | ||
| - | |||
| - | Upregulates inositol phosphate | ||
| - | |||
| - | Increases intracellular Ca2+ | ||
==Comparisons to other G proteins== | ==Comparisons to other G proteins== | ||
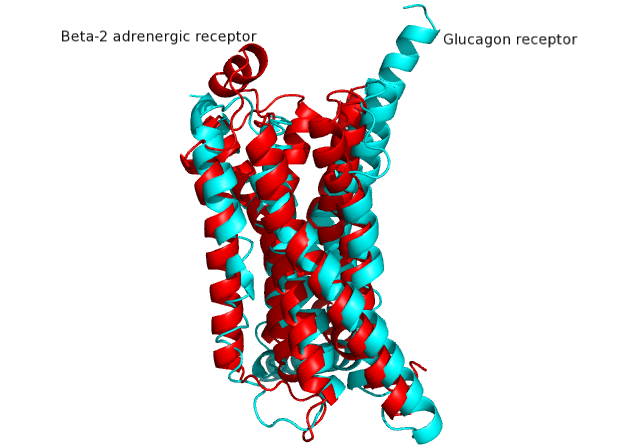
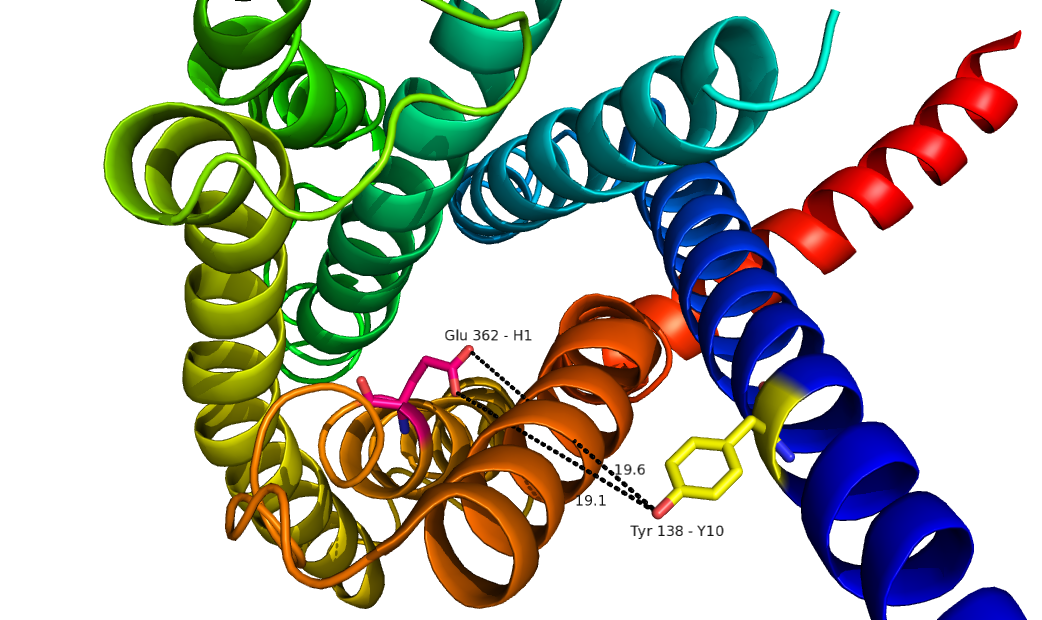
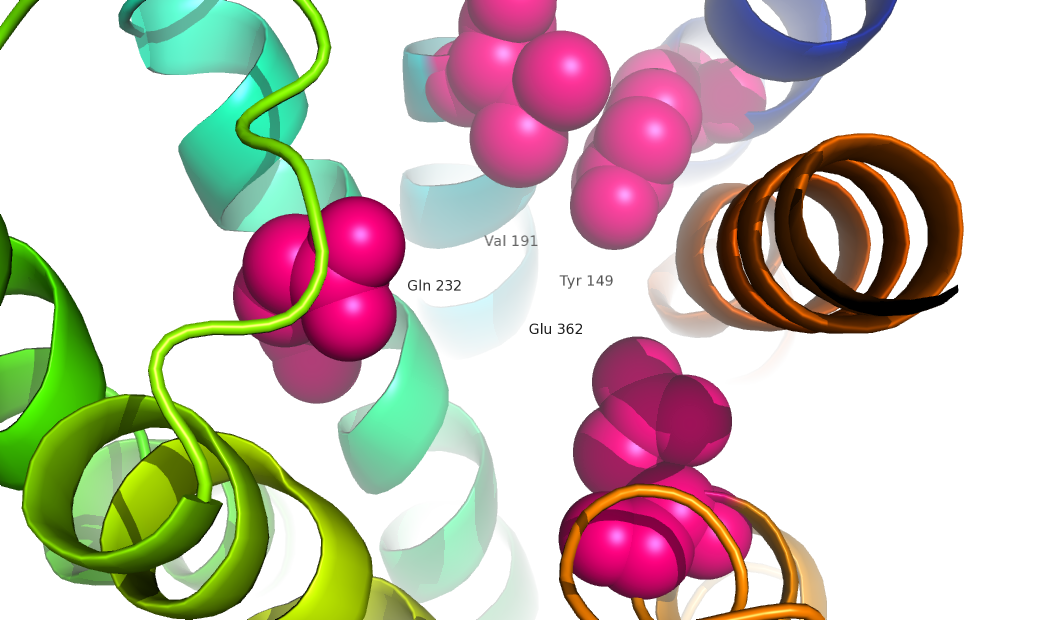
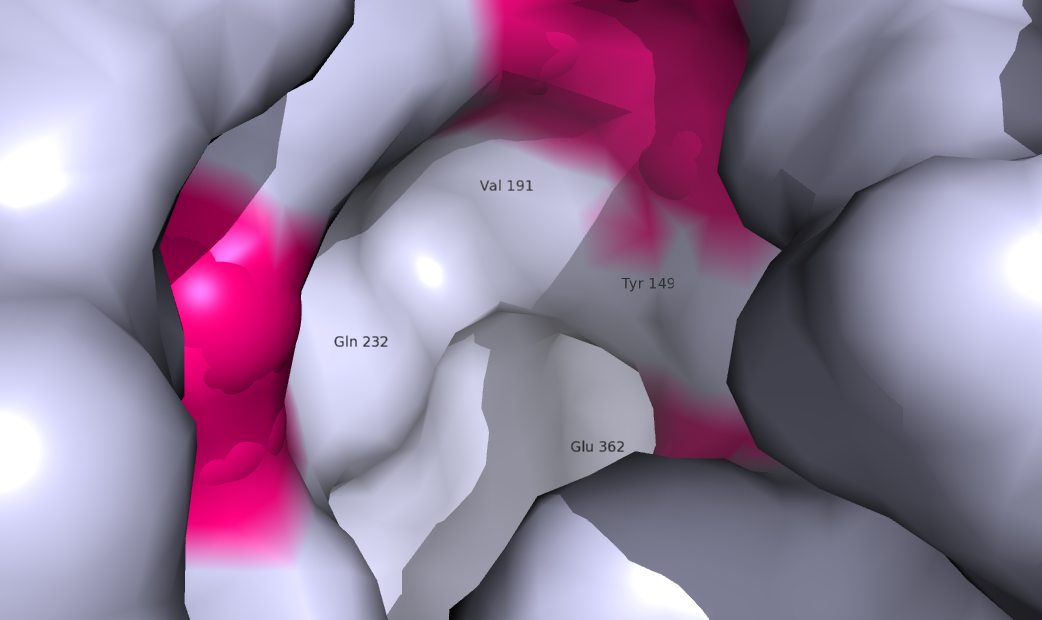
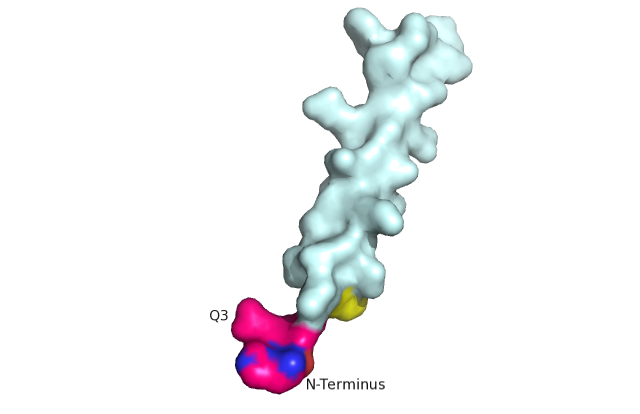
| + | GCGR is different from other Class A GPCRs in several ways. The first is that GCGR has a protrusion known as a 'stalk' which is three helical turn elongation of the N-terminus which protrudes past the extracellular (EC)membrane. ((CAN WE INSERT LINK TO 2D PIC HERE??)) Secondly, the extracellular loop 1 (ECL1) is 3-4 times longer than comparable loops in class A GPCRs. The stalk and ECL1 have been determined to affect ligand-receptor interaction. <ref>DOI 10.1038/nature12393</ref> | ||
===Structural similarities=== | ===Structural similarities=== | ||
Revision as of 02:02, 31 March 2016
Structure of Class B Human Glucagon G-Protein Coupled Receptors (GCGRs)
G protein coupled receptors (GPCRs) are recognized as the largest known class of integral membrane proteins and are divided into five families; the rhodopsin family (class A), the secretin family (class B), the adhesion family, the glutamate family (class C), and the frizzled/taste family (class F). Roughly 5% of the human genome encodes g-protein-coupled receptors which are responsible for the transduction of endogenous signals and the instigation of cellular response. The variants all contain a similar seven α-helical transmembrane domain (TMD) that, once bound to its peptide ligand, undergoes conformational change and tranduces a signal to coupled, heterotrimeric G proteins which initiate intracellular signal pathways and generate physiological and pathological processes. [1]
Class B GPCRs contain 15 distinct receptors for peptide hormones and generate their signal pathway through the activation of adenylate cyclase (AC) which increases concentration of cAMP, inositol phosphate, and calcium levels in cyto. [2] These signals are essential elements of intracellular signal cascades for human diseases including type II diabetes mellitus, osteoporosis, obesity, cancer, neurological degeneration, cardiovascular diseases, headaches, and psychiatric disorders; making their regulation through drug targeting of particular interest to companies developing novel molecules. [3] Structurally based approaches to the development of small-molecule agonists and antagonists have been hampered by the lack of accurate Class B TMD visualizations until recent crystal structures of corticoptropin-releasing factor receptor 1 and human glucagon were realized. [4] [5]
The glucagon class B GPCR (GCGR) is involved in glucose homeostasis through the binding of the signal peptide glucagon.
| |||||||||||
References
- ↑ Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput Biol. 2006 Feb;2(2):e13. Epub 2006 Feb 17. PMID:16485037 doi:http://dx.doi.org/10.1371/journal.pcbi.0020013
- ↑ Bortolato A, Dore AS, Hollenstein K, Tehan BG, Mason JS, Marshall FH. Structure of Class B GPCRs: new horizons for drug discovery. Br J Pharmacol. 2014 Jul;171(13):3132-45. doi: 10.1111/bph.12689. PMID:24628305 doi:http://dx.doi.org/10.1111/bph.12689
- ↑ Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014 Jan;35(1):12-22. doi: 10.1016/j.tips.2013.11.001. Epub, 2013 Dec 18. PMID:24359917 doi:http://dx.doi.org/10.1016/j.tips.2013.11.001
- ↑ Hollenstein K, Kean J, Bortolato A, Cheng RK, Dore AS, Jazayeri A, Cooke RM, Weir M, Marshall FH. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature. 2013 Jul 25;499(7459):438-43. doi: 10.1038/nature12357. Epub 2013 Jul 17. PMID:23863939 doi:http://dx.doi.org/10.1038/nature12357
- ↑ Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009 May;8(5):369-85. doi: 10.1038/nrd2782. Epub 2009 Apr, 14. PMID:19365392 doi:http://dx.doi.org/10.1038/nrd2782
- ↑ Xu Y, Xie X. Glucagon receptor mediates calcium signaling by coupling to G alpha q/11 and G alpha i/o in HEK293 cells. J Recept Signal Transduct Res. 2009 Dec;29(6):318-25. doi:, 10.3109/10799890903295150. PMID:19903011 doi:http://dx.doi.org/10.3109/10799890903295150
- ↑ Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ Weston C, Lu J, Li N, Barkan K, Richards GO, Roberts DJ, Skerry TM, Poyner D, Pardamwar M, Reynolds CA, Dowell SJ, Willars GB, Ladds G. Modulation of Glucagon Receptor Pharmacology by Receptor Activity-modifying Protein-2 (RAMP2). J Biol Chem. 2015 Sep 18;290(38):23009-22. doi: 10.1074/jbc.M114.624601. Epub, 2015 Jul 21. PMID:26198634 doi:http://dx.doi.org/10.1074/jbc.M114.624601