Sandbox Reserved 425
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
==Introduction== | ==Introduction== | ||
| - | Potatinib was developed as a treatment option for chronic myeloid leukemia (CML) as other inhibitors in treatment have become ineffective. Further mutations in BCR-ABL, a kinase with a cancerous genetic mutation in chromosome 22 that leaves it always active, has left earlier versions of tyrosine kinases unable to bind in almost 30% of cases over a course of 5 years of treatment. The mutant BCR-ABL kinase’s ability to develop new mutations has pushed for newer developments in inhibitiors like Potatinib | + | Potatinib was developed as a treatment option for chronic myeloid leukemia (CML) as other inhibitors in treatment have become ineffective. Further mutations in BCR-ABL, a kinase with a cancerous genetic mutation in chromosome 22 that leaves it always active, has left earlier versions of tyrosine kinases unable to bind in almost 30% of cases over a course of 5 years of treatment. The mutant BCR-ABL kinase’s ability to develop new mutations has pushed for newer developments in inhibitiors like Potatinib<ref name="seven">PMID: 21118377 </ref>. |
Fibroblast growth factor (FGFR) signaling, the factor that normally activates the BCR-ABL kinase, is the protein behind both tissue development and repair. The activation happens through a series of cascading signals that induce proliferation and migration in cells. Though mutations in the regulation of the FGFR tyrosine kinase family can result in malignant tumor growth<ref name="two" />. The tyrosine kinase inhibitor Ponatinib has been used to | Fibroblast growth factor (FGFR) signaling, the factor that normally activates the BCR-ABL kinase, is the protein behind both tissue development and repair. The activation happens through a series of cascading signals that induce proliferation and migration in cells. Though mutations in the regulation of the FGFR tyrosine kinase family can result in malignant tumor growth<ref name="two" />. The tyrosine kinase inhibitor Ponatinib has been used to | ||
<scene name='48/483882/Activation_loop/1'>bind</scene> to the mutant version of kinase BCR-ABL by the kinase's specific "DFG-out" conformation. The "DFG-out" conformation has the phenylalanine group of BCR-ABL flipped out of its hydrophobic binding site. Ponatinib is the first of its kind to be able to inhibit this specific mutation in BCR-ABL of the "DGF-out" combination<ref name="two" />. | <scene name='48/483882/Activation_loop/1'>bind</scene> to the mutant version of kinase BCR-ABL by the kinase's specific "DFG-out" conformation. The "DFG-out" conformation has the phenylalanine group of BCR-ABL flipped out of its hydrophobic binding site. Ponatinib is the first of its kind to be able to inhibit this specific mutation in BCR-ABL of the "DGF-out" combination<ref name="two" />. | ||
| - | The side effects of have caused Ponatinib to fall under scrutiny from the U.S. Food and Drug Administration (FDA). Ponatinib has shown to increase chances of deadly blood clotting and restenosis in both arteries and veins with a rate of about 1 in 5 patients. The drug has also shown to increase risk of heart attack and overall worsening of heart disease in patients | + | The side effects of have caused Ponatinib to fall under scrutiny from the U.S. Food and Drug Administration (FDA). Ponatinib has shown to increase chances of deadly blood clotting and restenosis in both arteries and veins with a rate of about 1 in 5 patients. The drug has also shown to increase risk of heart attack and overall worsening of heart disease in patients<ref name="seven" />. |
----------'''Notes (to be removed)''' | ----------'''Notes (to be removed)''' | ||
| Line 58: | Line 58: | ||
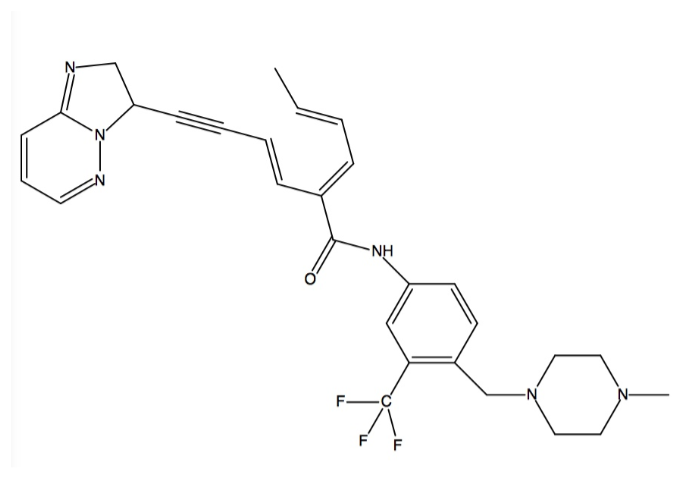
| - | Kinases are the largest drug targets currently being tested in clinical trials. All kinases possess a biolobal fold that is a smaller N-terminal and a larger C-terminal lobe joined together by a “hinge.” The cofactor ATP binds deeply into a pocket between the lobes and binds to the hinge region. The imposition of any other residue in this ATP-binding pocket controls access to the hydrophobic pocket by separating the adenine binding site from an adjacent hydrophobic pocket. Such residues are termed “gatekeepers,” and are critical considerations in the development of drugs to treat CML because of the mutations that these residues can ensue. Gatekeeper mutations that convert a small hydrophilic residue into a large hydrophobic residue are one example of what has been shown to result in drug resistance, specifically to the most well-known ABL inhibitors like imatinib (Gleevec) | + | Kinases are the largest drug targets currently being tested in clinical trials. All kinases possess a biolobal fold that is a smaller N-terminal and a larger C-terminal lobe joined together by a “hinge.” The cofactor ATP binds deeply into a pocket between the lobes and binds to the hinge region. The imposition of any other residue in this ATP-binding pocket controls access to the hydrophobic pocket by separating the adenine binding site from an adjacent hydrophobic pocket. Such residues are termed “gatekeepers,” and are critical considerations in the development of drugs to treat CML because of the mutations that these residues can ensue. Gatekeeper mutations that convert a small hydrophilic residue into a large hydrophobic residue are one example of what has been shown to result in drug resistance, specifically to the most well-known ABL inhibitors like imatinib (Gleevec)<ref name="four">PMID: 25317566</ref>. Ponatinib is a third generation type II pan-BCR-ABL kinase inhibitor, which allows it to bind even with the presence of gatekeeper mutations<ref name="five">PMID: 25219510</ref>. Type II inhibitors are classified by binding to the hydrophobic and allosteric pocket that is only accessible in the DFG-out conformation and that is next to the ATP binding pocket. Additionally, type II inhibitors extend deep into the adenine pocket and hydrogen bond with the hinge region<ref name="four" />. This unique ability is caused by ponatinib’s ability to overcome resistances of the BCR-ABL gatekeeper mutant T315I at low concentrations (low IC50s ranging from 0.5 nM to 36 nM) by an ethynyl linker in the <scene name='48/483882/Active_site1/1'>DFG-out</scene> conformation [5, 6]. The T315I mutation accounts for 15-20% of all clinically observed mutations and it is resistant to all previous generation drugs (imatinib, nilotinib, dasatinib). Additionally, ponatinib has a very high potency against native ABL which allows the binding energy to be distributed over many protein residues<ref name="five">. |
| - | The specific binding of ponatinib can be categorized into and explained by five major chemical components, they are: (1) the template that interacts with the hinge region; (2) the A ring that occupies the hydrophobic pocket behind the gatekeeper residue; (3) the key ethynyl linker that joins the template and A ring, and that interacts with the gatekeeper residue (linker 1); (4) the A-B ring linker (linker 2); and (5) the B ring that binds to the DFG-out pocket | + | The specific binding of ponatinib can be categorized into and explained by five major chemical components, they are: (1) the template that interacts with the hinge region; (2) the A ring that occupies the hydrophobic pocket behind the gatekeeper residue; (3) the key ethynyl linker that joins the template and A ring, and that interacts with the gatekeeper residue (linker 1); (4) the A-B ring linker (linker 2); and (5) the B ring that binds to the DFG-out pocket<ref name="five">. |
| - | Ponatinib binds into the ATP binding pocket between the N and C lobes to induce a shift from the DFG-in to the DFG-out conformation. It covers an immense region that spans from the kinase hinge region (back of kinase) to the catalytic pocket (front of kinase). Three sites are engaged in the ATP binding cleft by ponatinib’s aromatic rings. In the first site, the imidazo[1,2b]pyridazine scaffold takes up the same space as the adenine ring of ATP and it is able to form one hydrogen bone with the backbone amide nitrogen atom of Ala553 in the hinge | + | Ponatinib binds into the ATP binding pocket between the N and C lobes to induce a shift from the DFG-in to the DFG-out conformation. It covers an immense region that spans from the kinase hinge region (back of kinase) to the catalytic pocket (front of kinase). Three sites are engaged in the ATP binding cleft by ponatinib’s aromatic rings. In the first site, the imidazo[1,2b]pyridazine scaffold takes up the same space as the adenine ring of ATP and it is able to form one hydrogen bone with the backbone amide nitrogen atom of Ala553 in the hinge<ref name="seven" />. Both of its rings form several van der Waals contacts with residues in the N and C lobes of the adenine binding site as well<ref name="five">. Rigid acetylene linkage drives the rest of the drug into the back of the ATP binding pocket. In the second site, the methylphenyl group displaces the side chain of the catalytic Lys503 and its aromatic ring binds to the hydrophobic pocket that is formed by Val550, the gatekeeper residue, and Met524<ref name="seven" />. Val550 is stabilized by the benzimide group<ref name="six">PMID: 25478866</ref>. This displacement allows Glu520 in the αC helix to hydrogen bond with the amide linkage between the aromatic rings in ponatinib. In the third site, Phe631 of DFG is expelled out of the cleft by ponatinib’s 3-trifluoromethylphenyl moiety, which takes the place of Phe631. Phe631’s new position enables it to make hydrophobic contact with the drug’s scaffold and acetylene linker. Also, Asp630 of DFG becomes available for hydrogen bonding with the amide linkage between the aromatic rings in ponatinib. This also puts the piperazine ring in the position to engage in hydrogen bonding with the catalytic loop. This is shift forms the DFG-out conformation<ref name="seven" />. |
| - | Other inhibitors are not as potent as ponatinib against FGFR kinases because they are unable to penetrate far enough to access the third site and cannot assume the DFG-out conformation | + | Other inhibitors are not as potent as ponatinib against FGFR kinases because they are unable to penetrate far enough to access the third site and cannot assume the DFG-out conformation<ref name="seven" />. |
==Additional Features== | ==Additional Features== | ||
| - | Ponatinib is an orally ingested tyrosine kinase inhibitor that has shown promising avenues of treatment for counteracting the effects of angiogenesis in tumor growth. Besides the inhibition of FGFRs, this agent inhibits tyrosine kinases involved in vascular endothelial growth factor receptors. Ponatinib is considered a third generation TKI that can treat even the most drug-therapy resistant mutations that previous TKIs were incapable of treating | + | Ponatinib is an orally ingested tyrosine kinase inhibitor that has shown promising avenues of treatment for counteracting the effects of angiogenesis in tumor growth. Besides the inhibition of FGFRs, this agent inhibits tyrosine kinases involved in vascular endothelial growth factor receptors. Ponatinib is considered a third generation TKI that can treat even the most drug-therapy resistant mutations that previous TKIs were incapable of treating<ref name="eight">PMID: 23986642</ref>. |
| - | The brand name for ponatinib is Iclusig. Iclusig received an accelerated approval grant through the Food and Drug Administration. It was mainly prescribed to patients suffering from CML or ALL who did not make any progress with the first and second generation TKIs. However, the clinical trials data revealed a spike in adverse effects. These consequences include heart failure, stroke, coronary artery disease, loss of blood flow to body parts leading to amputation amongst other narrowing of blood vessels[ | + | The brand name for ponatinib is Iclusig. Iclusig received an accelerated approval grant through the Food and Drug Administration. It was mainly prescribed to patients suffering from CML or ALL who did not make any progress with the first and second generation TKIs. However, the clinical trials data revealed a spike in adverse effects. These consequences include heart failure, stroke, coronary artery disease, loss of blood flow to body parts leading to amputation amongst other narrowing of blood vessels<ref>FDA Drug Safety Communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins; Drug Safety and Availability; United States Food and Drug Administration (2013). Web. [http://www.fda.gov/Drugs/DrugSafety/ucm370945.htm]</ref>. |
| Line 117: | Line 117: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| - | |||
| - | [4] Huang, Zhifeng et al. "DFG-Out Mode Of Inhibition By An Irreversible Type-1 Inhibitor Capable Of Overcoming Gate-Keeper Mutations In FGF Receptors". ACS Chem. Biol. 10.1 (2015): 299-309. Web. | ||
| - | |||
| - | [5] Lesca, E. et al. "Structural Analysis Of The Human Fibroblast Growth Factor Receptor 4 Kinase". Journal of Molecular Biology 426.22 (2014): 3744-3756. Web. | ||
| - | |||
| - | [6] Vijayan, R. S. K. et al. "Conformational Analysis Of The DFG-Out Kinase Motif And Biochemical Profiling Of Structurally Validated Type II Inhibitors". J. Med. Chem. 58.1 (2015): 466-479. Web. | ||
| - | |||
| - | [7] Zhou, Tianjun et al. "Structural Mechanism Of The Pan-BCR-ABL Inhibitor Ponatinib (AP24534): Lessons For Overcoming Kinase Inhibitor Resistance". Chemical Biology & Drug Design 77.1 (2010): 1-11. Web. | ||
| - | |||
| - | [8]Price KE, Saleem N, Lee G, Steinberg M. Potential of ponatinib to treat chronic myeloid leukemia and acute lymphoblastic leukemia. OncoTargets and therapy. 2013;6:1111-1118. doi:10.2147/OTT.S36980. | ||
| - | |||
| - | [9]FDA Drug Safety Communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins; Drug Safety and Availability; United States Food and Drug Administration (2013). Web. | ||
Revision as of 17:31, 6 April 2016
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Fibroblast Growth Factor Receptor/Ponatinib (4uxq) [1]
by Julie Boshar, Emily Boyle, Nicole Kirby, Cory Thomas, Connor Walsh
Student Projects for UMass Chemistry 423 Spring 2016
| |||||||||||