Sandbox Reserved 1160
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
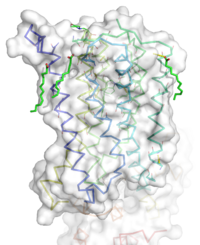
[[Image:STR.png|200 px|left|thumb|'''Figure 1''': Overall Structure of the mGlu5 TMD. The polar heads on the Oliec acids orient the image with the top oriented extracellularly, the middle portion inserted into the membrane, and the lower portion oriented intracellularly. The white exterior represents the surface of the protien, and the multicolored lines interior to the surface represent the backbones 7 transmembrane alpha helices. ]] | [[Image:STR.png|200 px|left|thumb|'''Figure 1''': Overall Structure of the mGlu5 TMD. The polar heads on the Oliec acids orient the image with the top oriented extracellularly, the middle portion inserted into the membrane, and the lower portion oriented intracellularly. The white exterior represents the surface of the protien, and the multicolored lines interior to the surface represent the backbones 7 transmembrane alpha helices. ]] | ||
=== Overview === | === Overview === | ||
| - | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/3'> alpha helices</scene> that spans the membrane. The protein was crystallized with Oleic acid | + | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/3'> alpha helices</scene> that spans the membrane. The protein was crystallized with <scene name='72/721531/Protien_clean_sce/2'>Oleic acid</scene>. On the top portion of the protein several critical extracellular loops connect the TMD to the CRD. The binding pocket can be found near the middle of the protein, and is mainly comprised of hydrophobic amino acids with more polar amino acids found in the upper and lower portions of the binding site. Inserted into the biding pocket is the negative allosteric modulator [http://www.en.wikipedia.org/wiki/Mavoglurant mavoglurant]. The TMD is in an inactive conformation, since mavoglurant is bound. Also, the deletion of the flexible domains leaves the mGlu5 receptor unable to bing to its [[GPCR]]. The inactive state is maintained by multiple ionic locks whose positions determine the active versus inactive conformation. |
=== Extracellular Domain === | === Extracellular Domain === | ||
These are the <scene name='72/721532/Ecl_trail_1/7'>Extracellular Loops</scene> with extracellular loops (ECLs) 1, 2, and 3 highlighted in purple. Additionally in the ECL domain, a <scene name='72/721532/Ecl_trail_1/6'>disulfide bond</scene> is attached to both Helix 3 and the amino acid chain between Helix 5 and the N terminus. The disulfide bond is highlighted in yellow, and it is conserved in all classes of glutamate receptor 5 transmembrane domains<ref name="Wu" />. | These are the <scene name='72/721532/Ecl_trail_1/7'>Extracellular Loops</scene> with extracellular loops (ECLs) 1, 2, and 3 highlighted in purple. Additionally in the ECL domain, a <scene name='72/721532/Ecl_trail_1/6'>disulfide bond</scene> is attached to both Helix 3 and the amino acid chain between Helix 5 and the N terminus. The disulfide bond is highlighted in yellow, and it is conserved in all classes of glutamate receptor 5 transmembrane domains<ref name="Wu" />. | ||
=== Binding Pocket === | === Binding Pocket === | ||
[[Image: Organic with clipped surface.png|200 px|left|thumb|Cross section view of mavoglurant in the binding pocket]] | [[Image: Organic with clipped surface.png|200 px|left|thumb|Cross section view of mavoglurant in the binding pocket]] | ||
| - | The binding pocket represents an | + | The binding pocket, not to be confused with the glutamate binding site, represents an useful source of regulatory control. The binding pocket is only accessible by a relatively narrow (7 Å) <scene name='72/721531/Protien_sur/4'>entrance</scene><ref name="Dore" />. This small entrance severely restricts the access of both positive and negative allosteric regulators. |
One such regulator, mavoglurant, is a | One such regulator, mavoglurant, is a | ||
Important Amino Acids<ref name="Dore" />: | Important Amino Acids<ref name="Dore" />: | ||
*<scene name='72/721531/Protien_bindtop/4'>Asparagine</scene> 747 forms a hydrogen bond network with the main chain carbonyl of Glycine 652 and the carbamate portion of mavoglurant. | *<scene name='72/721531/Protien_bindtop/4'>Asparagine</scene> 747 forms a hydrogen bond network with the main chain carbonyl of Glycine 652 and the carbamate portion of mavoglurant. | ||
| - | *Bicyclic ring surrounded by <scene name='72/721531/Protien_hydrophobic/1'>hydrophobic binding pocket</scene>. | + | *Bicyclic ring of mavoglurant surrounded by <scene name='72/721531/Protien_hydrophobic/1'>hydrophobic binding pocket</scene>. |
| - | *2 Catalytic <scene name='72/721531/Protien_bindmiddle/2'>serine</scene> | + | *2 Catalytic <scene name='72/721531/Protien_bindmiddle/2'>serine</scene> residues H-bond to the hydroxyl oxygen of our ligand. |
*A <scene name='72/721531/Protien_bindbottom/1'>water molecular</scene> inside of the binding pocket helps stabilize the inactive state. | *A <scene name='72/721531/Protien_bindbottom/1'>water molecular</scene> inside of the binding pocket helps stabilize the inactive state. | ||
| - | Once bound to mavoglurant, transmembrane helix 7 undergoes a conformational change<ref name="Dore" />. The shifting of TM7 will lead to a more global conformational change, which | + | Once bound to mavoglurant, transmembrane helix 7 undergoes a conformational change<ref name="Dore" />. The shifting of TM7 will lead to a more global conformational change, which inactivates the receptor<ref name="Dore" />. Variation can be seen in positioning of alpha helices across receptor class. Class C receptors have seemingly less space for mavoglurant to enter as compared to Class A and F receptors<ref name="Wu" />. The position of the ligand binding site is also varied between different classes of mGlu receptors<ref name="Dore" />. |
=== Ionic Locks === | === Ionic Locks === | ||
| - | Another important structural feature is the series of <scene name='72/721531/Ionic_lock/4'>ionic locks</scene> on the intracellular side of the protein. Interactions between amino acids will form a salt bridge, which will stabilize the inactive conformation<ref name="Dore" />. The primary ionic lock forms between Glu770, Lys665, and Ser613<ref name="Dore" />. A secondary ionic lock occurs between Ser614 and Arg668<ref name="Dore" />. The purpose of these ionic locks is analogous to the ionic interactions that stabilize the T state in [[Hemoglobin]]. In the case of the TMD, when the NAM mavoglurant is bound | + | Another important structural feature is the series of <scene name='72/721531/Ionic_lock/4'>ionic locks</scene> on the intracellular side of the protein. Interactions between these five amino acids will form a salt bridge, which will stabilize the inactive conformation<ref name="Dore" />. The primary ionic lock forms between Glu770, Lys665, and Ser613<ref name="Dore" />. A secondary ionic lock occurs between Ser614 and Arg668<ref name="Dore" />. The purpose of these ionic locks is analogous to the ionic interactions that stabilize the T state in [[Hemoglobin]]. In the case of the TMD of mGlu5, the ionic lock is formed when the NAM mavoglurant is bound. This ionic lock stabilizes the inactive state, where the intracellular loops are stabilized facing inwards<ref name="Wu" />. This conformational change will effectively block the crevice that is involved in binding the G-protein<ref name="Wu" />. Models have suggested that, even in a glutamate bound state, the mavoglurant bound receptor would be dimerized but incapable of signaling<ref name="Wu" />. This signaling incapable mGlu5 dimer will help maintain the readiness of the pathway, while still decreasing signal response. |
== Pathway == | == Pathway == | ||
Revision as of 19:16, 10 April 2016
Human metabotropic glutamate receptor 5 transmembrane domain
| |||||||||||