Introduction
The (VDR) is a ligand-dependent transcriptional regulator with two strands. VDR belongs to the superfamily of nuclear receptors which control homeostasis, cell differentiation and growth, and many physiological processes. All proteins that belong to the nuclear receptor superfamily have a variable N-terminus region (A/B region), a hinge region that is flexible (D region), a conserved DNA-binding region (DBD, C region), and a moderately conserved ligand-binding region (LBD, E/F region). In the case of VDR, the A/B region is very short so it does not have any AF-1 function and the ligand binding region has a dimerization interface and a transcriptional activation domain that is ligand-dependent (AF-2).[1]
The VDR has both an active and suppressed form. The activation or suppression function is caused by the binding of the DR3 response element as a heterodimer with the retinoid X receptor of the target genes. Due to the interactions with the basal transcriptional machinery and transcriptional cofactors, transcription is either activated or suppressed. When VDR is in its active form it regulates both phosphate and calcium metabolism, has immunosuppressive effects, and induces cell differentiation. When there are defects in the VDR that effect its metabolism it can lead to diseases such as severe rickets, secondary hyperparathyroidism, and hypocalcemia. Though defects in VDR can cause many diseases, fully functioning VDR can be used as treatment for disease such as cancer, autoimmune disease, psoriasis, osteoporosis, and renal osteodystrophy.[1]
Overall Structure
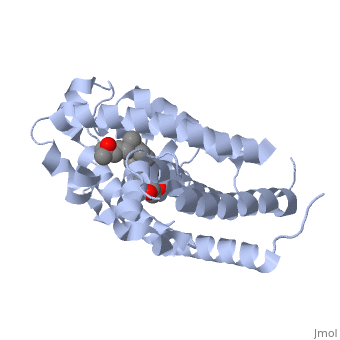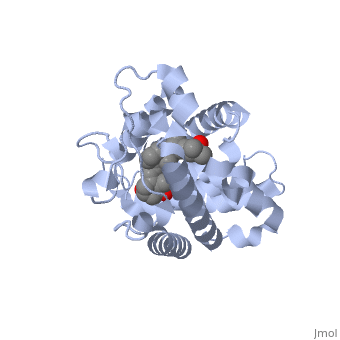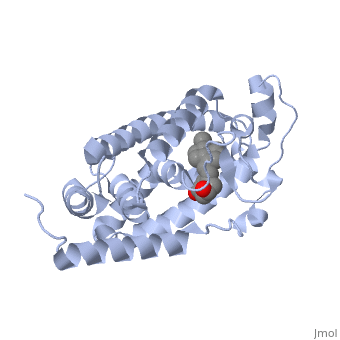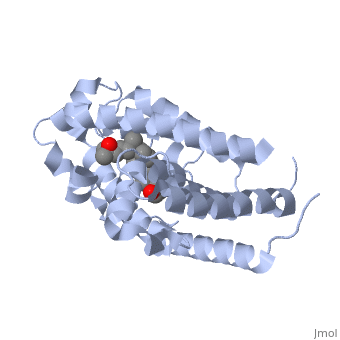
The contains 427 amino acids with a total molecular weight of 48,289 Da. The protein is also composed almost entirely of alpha helices with only a single beta sheet. The vitamin D receptor also does not have a quaternary structure [2]. is a large organic compound made up of 27 carbon atoms, 44 hydrogen atoms and a single oxygen atom, with the ligand having a total molecular weight of 385 Da [3]. In studying the vitamin d receptor, the regions of the protein have been categorized into domains, with the A/B domain located at the N-terminus, the C domain, which is located between amino acid 20 and amino acid 115, the D domain, which is located between the end of the C domain and amino acid 220, and the EF domain, which encompasses the rest of the protein [4].
Other aspects of interest about the vitamin D receptor include the protein revealing a binding pocket when it is in its active folded state, allowing the ligand to bind to the receptor. The ligand interacts with the activation helix by stabilizing the agonist position. This is accomplished through Van der Waals interactions between the ligand and the activation helix. The activation ligand is a nuclear receptor. There is also some empty space observed around the aliphatic chain [1].
The protein has an active conformation of 1,25 (OH)_2D_3 that has a ligand binding pocket in its active folded state. The activation ligand, a nuclear receptor (VDR), interacts with with the activation helix by stabilizing the agonist position. This is accomplished through Van der Waals interactions between the ligand and the activation helix. There is some empty space observed around the aliphatic chain, indicating the presence of water to stabilize all possible hydrogen bonds [1].
Binding Interactions
Protein 1db1 is found to complex with 1,25 Dihydroxy . This molecule has three notable alcohol groups shown in red. Oxygen is electronegative, giving alcohols the ability to participate in hydrogen bonding with the protein. Vitamin D3 has a large number of relations with the residues on the protein chain. First in the sequence are . Tyr143, shown in blue, is the closest to the ligand at 2.83 angstroms. This is sightly large but there is still the possibility of hydrogen bonding. Tyr147 in green and Phe150 in black are also known to have interactions with vitamin D3 they are farther away and therefore less significant. Next down the peptide chain are . Ser237, shown in green, has significant interactions with vitamin D3 this can be seen by its short distance 2.78 angstroms. Only 40 residues away, more hydrogen bonding is occurring. form bonds with the same oxygen atoms as Tyr143 and Ser237 respectively. This means that the oxygen atoms of vitamin D3, when bound to the receptor, are negatively charged and stabilized by protons. On the opposite end of vitamin D3 there is an additional negatively charged oxygen. Although this oxygen does not participate in hydrogen bonding. , shown in blue and green respectively, Contain aromatic rings. These rings are able to momentarily accept the electrons donated by the oxygen because they can delocalize the charge. This creates two pseudo-covalent bonds that is approximately 2.81 angstroms. When looking at , it can be seen that all of the binding sights are centered around oxygen [5,6].
Additional Features
Hereditary Vitamin D Resistant Rickets (HVDRR) is a condition that most commonly occurs in children and results in soft and weak bone formation, which often causes deformities in bone structure. A lack of proper nutrients, Vitamin D3 in particular, as well as defects in the Vitamin D receptor can cause rickets in humans. HVDRR can occur when the VDR is impaired in its ability to activate transcription in response to the 1,25-(OH)2D3 ligand [7]. VDR regulates the hormonal form of Vitamin D, through modifying the transcription of the target to a certain sequence of DNA, called the Vitamin D responsive element (VDRE). This activation requires an additional receptor, which is a Retinoid X Receptor (RXR) to bind to the heterodimer [7]. A mutation in the transcription of the protein has the potential to result in this disease and the mutation results in the not forming properly. Current research shows that there are two mechanisms that can cause this failed transcription and inability to bind to the heterodimer to occur within the body. The first is a point mutation of an amino acid in the zinc finger region of the VDR that reduces the binding of the heterodimer, this region is found in the amino acid residues 21-85 [7]. The other is a premature stop codon in the DNA sequences that does not allow for the full transcription, which can have an effect of reducing the affinity of the heterodimer binding [7]. Although there may be other mutations that could cause HVDRR to occur within the body, these two types of mutations have been shown to be major causes of the disease.
Also, the VDR has an effect on the hair follicle cycle, which has been observed through the eliminating the expression of this receptor. In null-VDR mice, it has been shown that with normal mineral ion levels that the mice result in alopecia, a disease that causes hair loss [8]. VDR is expressed in the hair follicle keratinocytes and its levels are higher in the late anagen and catagen stages of the hair cycle [8]. These two stages are vital in the differentiation and proliferation of hair follicle keratinocytes, which regulate hair growth in the body. Much research has been done into the mechanism in which the VDR effects the hair follicle cycle with the overall mechanism still unknown. The mechanism was first believed to be that of binding the VDR to 1,25- dihydroxyvitamin D causing transactivation due to the fact that targeted expressions of wild-type VDR to the keratinocytes of VDR null mice rescued alopecia [8]. Although, this was disproven through investigations in vitamin D3-deficient mice that had no detectable 1,25-dihydroxyvitamin D for the VDR to bind, yet the mice did not develop alopecia. This shows that the VDR transcriptional activation of DNA is not the main cause of the loss of hair follicles. Current research observes the ligand-independent actions of the VDR that have not been observed extensively as a mechanism [8]. Nuclear receptor co-repressor genes have been observed in studies to have an effect on the hair follicle cycle including the HR gene (Hairless). This corepressor has been shown to have interactions with the VDR in vivo and tests with the mutation of Hairless have caused alopecia in mice in vivo [8]. Thus, although the mechanism behind the interaction of Hairless and the VDR is still unknown it has been shown in studies that there is a relationship between the two in the body and that it has been linked to the hair follicle cycle.
Quiz Question 1
Osteoporosis is a disease in which the bones become porous and fragile. The most common cause of the ailment is calcium deficiency. As the vitamin D receptor has association with calcium uptake, mutations in VDR could be detrimental.If an individual had a point mutation that would replace with serine amino groups, would that individual be more or less likely to develop osteoporosis? why?
See Also
Credits
Introduction - Kate Daborowski
Overall Structure - Aaron Thole and Benjamin Rizkin
Drug Binding Site - Roger Crocker
Additional Features - Patrick Murphy
Quiz Question 1 - Roger Crocker
References
- ↑ Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000 Jan;5(1):173-9. PMID:10678179
1. Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000 Jan;5(1):173-9.
2. VDR Gene http://www.genecards.org/cgi-bin/carddisp.pl?gene=VDR (accessed Apr 2, 2016).
3. Vitamin D3 https://pubchem.ncbi.nlm.nih.gov/compound/Vitamin_D3#section=2D-Structure (accessed Apr 10, 2016).
4. Strugnell, S.; Deluca, H. The Vitamin D Receptor - Structure and Transcriptional Activation. Experimental Biology and Medicine1997, 215, 223–228.
5. Vitamin D Receptor http://pdb101.rcsb.org/motm/155 (accessed Apr 4, 2016).
6. 3D Binding Pocket http://www.rcsb.org/pdb/explore/jmol.do?structureId=1DB1&residueNr=VDX (accessed Apr 4, 2016).
7. Whitfield, G.K.; Selznick, S.H.; Haussler, C.A.; Hsieh, J.; Galligan, M.A.; Jurutka, P.W.; Thompson, P.D.; Lee, S.M.; Zerwekh, J.E.; Haussler, M.R. Vitamin D Receptors from Patients with Resistance to 1,25-Dihydroxyvitamin D3: Point Mutations Confer Reduced Transactivation in Response to Ligand and Impair Interaction with the Retinoid X Receptor Heterodimeric Partner. Mol Endocrinol, 1996 10 (12): 1617-1631
8. Skorija, K.; Cox, M.; Sisk, J.M.; Dowd, D.R.; MacDonald, P.N.; Thompson, C.C.; Demay, M.B. Ligand- Independent Actions of the Vitamin D Receptor Maintain Hair Follicle Homeostasis. Mol Endocrinol, April 2005, 19(4):855–862