User:Brittany Stankavich/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
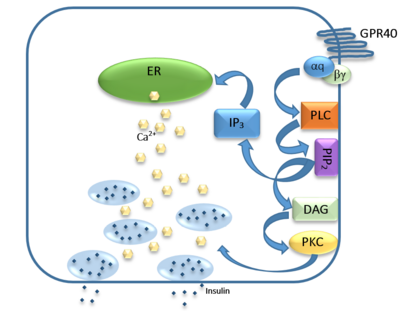
The natural substrate of hGPR40 are free fatty acids (FFAs) which bind to the G-protein and enhance glucose-stimulated insulin secretion. FFAs bind to GPR40 which then couples with the G-protein Gq leading to increased [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] (PLC) activity. PLC catalyzes the hydrolysis of the phospholipid [https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate phosphatidylinositol-4,5-biphosphate ] (PIP<sub>2</sub>) resulting in the formation of [https://en.wikipedia.org/wiki/Diglyceride diacylglycerol] (DAG) and [https://en.wikipedia.org/wiki/Inositol_trisphosphate inositol 1,4,5-triphosphate] (IP3). DAG can activate [https://en.wikipedia.org/wiki/Protein_kinase_C protein kinase C] (PKC) to enhance insulin secretion. IP3 on the other hand is soluble and diffuses to the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum] where it is able to bind to a receptor on a ligand-gated Ca<sup>2+</sup> channel. This binding triggers the opening of the channel causing stored Ca<sup>2+</sup> to be released into the cytoplasm. Upon this large increase in intracellular free Ca<sup>2+</sup>, there is also an increase in glucose-dependent insulin secretion suggesting that insulin release can be contributed in part to the changes in Ca<sup>2+</sup> concentration resulting from activated GPR40 <ref name="Morg">PMID:19660440</ref>. | The natural substrate of hGPR40 are free fatty acids (FFAs) which bind to the G-protein and enhance glucose-stimulated insulin secretion. FFAs bind to GPR40 which then couples with the G-protein Gq leading to increased [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] (PLC) activity. PLC catalyzes the hydrolysis of the phospholipid [https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate phosphatidylinositol-4,5-biphosphate ] (PIP<sub>2</sub>) resulting in the formation of [https://en.wikipedia.org/wiki/Diglyceride diacylglycerol] (DAG) and [https://en.wikipedia.org/wiki/Inositol_trisphosphate inositol 1,4,5-triphosphate] (IP3). DAG can activate [https://en.wikipedia.org/wiki/Protein_kinase_C protein kinase C] (PKC) to enhance insulin secretion. IP3 on the other hand is soluble and diffuses to the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum] where it is able to bind to a receptor on a ligand-gated Ca<sup>2+</sup> channel. This binding triggers the opening of the channel causing stored Ca<sup>2+</sup> to be released into the cytoplasm. Upon this large increase in intracellular free Ca<sup>2+</sup>, there is also an increase in glucose-dependent insulin secretion suggesting that insulin release can be contributed in part to the changes in Ca<sup>2+</sup> concentration resulting from activated GPR40 <ref name="Morg">PMID:19660440</ref>. | ||
| + | |||
| + | == Structure == | ||
| + | |||
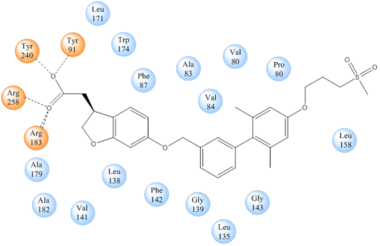
| + | hGPR40 is composed of seven-transmembrane helices that are characteristic of G-protein coupled receptors (GPCR). While there is relatively low sequence identity between hGPR40 and peptide-binding GPCRs and opioid receptors, they do share structural similarities such as a conserved hairpin loop motif on extracellular loop 2 (ECL2) as well as a disulphide bond that is formed between transmembrane helix 3 and the C-terminus of ECL2. Compared to peptide-binding and opioid GPCRs which have distinctive B-sheets spanning from transmembrane helix 4 to 5, hGPR40 possesses a shorter B-sheet-like region which has low B-factors. This reflects the low mobility of the region that limits the overall flexibility of the adjacent portion of ECL2 between Leu171 and Asp175 which forms a cap over the canonical binding site covering it from the central extracellular region. | ||
== Ligand Binding == | == Ligand Binding == | ||
Revision as of 01:14, 20 April 2016
- User:Brittany Stankavich/Sandbox 1
hGPR40 Homo sapiens
| |||||||||||
References
- ↑ Ren XM, Cao LY, Zhang J, Qin WP, Yang Y, Wan B, Guo LH. Investigation of the Binding Interaction of Fatty Acids with Human G Protein-Coupled Receptor 40 Using a Site-Specific Fluorescence Probe by Flow Cytometry. Biochemistry. 2016 Mar 17. PMID:26974599 doi:http://dx.doi.org/10.1021/acs.biochem.6b00079
- ↑ Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):82-8. doi:, 10.1016/j.prostaglandins.2009.05.003. Epub 2009 May 19. PMID:19460454 doi:http://dx.doi.org/10.1016/j.prostaglandins.2009.05.003
- ↑ 3.0 3.1 3.2 Morgan NG, Dhayal S. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem Pharmacol. 2009 Dec 15;78(12):1419-27. doi: 10.1016/j.bcp.2009.07.020., Epub 2009 Aug 4. PMID:19660440 doi:http://dx.doi.org/10.1016/j.bcp.2009.07.020
- ↑ Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012 Feb 17;335(6070):851-5. PMID:22344443 doi:10.1126/science.1215904
- ↑ Li X, Zhong K, Guo Z, Zhong D, Chen X. Fasiglifam (TAK-875) Inhibits Hepatobiliary Transporters: A Possible Factor Contributing to Fasiglifam-Induced Liver Injury. Drug Metab Dispos. 2015 Nov;43(11):1751-9. doi: 10.1124/dmd.115.064121. Epub 2015, Aug 14. PMID:26276582 doi:http://dx.doi.org/10.1124/dmd.115.064121
- ↑ 6.0 6.1 Takano R, Yoshida M, Inoue M, Honda T, Nakashima R, Matsumoto K, Yano T, Ogata T, Watanabe N, Hirouchi M, Yoneyama T, Ito S, Toda N. Discovery of DS-1558: A Potent and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2015 Jan 13;6(3):266-70. doi: 10.1021/ml500391n. eCollection, 2015 Mar 12. PMID:25815144 doi:http://dx.doi.org/10.1021/ml500391n