This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
TEM1 Class Antibiotic Resistance Proteins
From Proteopedia
| Line 15: | Line 15: | ||
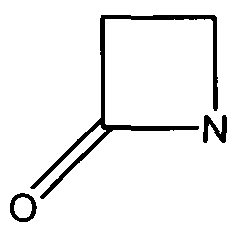
Figure 1. The Beta-Lactam ring is shown above. Beta-lactamases function in hydrolyzing the amide bond within the ring, rendering the antibiotic ineffective. (Image Credit: Wikipedia) | Figure 1. The Beta-Lactam ring is shown above. Beta-lactamases function in hydrolyzing the amide bond within the ring, rendering the antibiotic ineffective. (Image Credit: Wikipedia) | ||
| + | |||
β-Lactamases function by hydrolyzing the β-lactam ring within the antibiotic (Figure 2). This prevents the interaction between the cell wall and the antibiotic. | β-Lactamases function by hydrolyzing the β-lactam ring within the antibiotic (Figure 2). This prevents the interaction between the cell wall and the antibiotic. | ||
| Line 21: | Line 22: | ||
Figure 2. Bacteria are capable of becoming antibiotic resistant by catalyzing the hydrolysis of the β-lactam ring. | Figure 2. Bacteria are capable of becoming antibiotic resistant by catalyzing the hydrolysis of the β-lactam ring. | ||
| + | |||
The TEM-1 subclass is one of the most common of all β-Lactamases.[3] Their prevalence began when bacteria started exhibiting penicillin resistance on a mass scale. Since the TEM-1 subclass has been prevalent for many years, many inhibitors have either been discovered or synthesized to prevent their catalytic function. | The TEM-1 subclass is one of the most common of all β-Lactamases.[3] Their prevalence began when bacteria started exhibiting penicillin resistance on a mass scale. Since the TEM-1 subclass has been prevalent for many years, many inhibitors have either been discovered or synthesized to prevent their catalytic function. | ||
Revision as of 20:06, 17 April 2016
| |||||||||||
References
1. Davies, J.; Davies, G. Origins and Evolution of Antibiotic Resistance. Microbiol Mol Biol Rev. 2010, Sep; 74(3): 417–433.
2. National Institute of Health. Stop the Spread of Superbugs Help Fight Drug-Resistant Bacteria. https://newsinhealth.nih.gov/issue/feb2014/feature1. (Last accessed: April 11, 2016).
3. Dablon et al. The catalytic mechanism of f3-lactamases: NMR titration of an active-site lysine residue of the TEM-1 enzyme. Proc. Natl. Acad. Sci. USA. 1996, 74: 1747-1752.
4. Fonze, E.; Charlier, P.; To'th, Y.; Vermeire, M.; Raquet, X.; Dubus, A.; Frere, J. M. TEM1 beta-lactamase structure solved by molecular replacement and refined structure of the S235A mutant.
Acta Crystallogr. D Biol. Crystallogr. 1995, 51, 682-694.
5. Lenfant, F.; Labia, R.; Masson, J. -. Replacement of lysine 234 affects transition state stabilization in the active site of ß-lactamase TEM1. J. Biol. Chem. 1991, 266, 17187-17194.
Proteopedia Page Contributors and Editors (what is this?)
Kenna Salvatore, Matt O'Malley, Ryan Hunter Wilson, Riley Culhane, Michal Harel