We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1167
From Proteopedia
(Difference between revisions)
| Line 45: | Line 45: | ||
== Glucagon Binding == | == Glucagon Binding == | ||
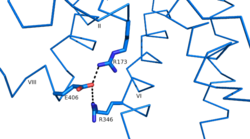
| - | Research has shown that class B GCPRs exist in either an [http://www.nature.com/ncomms/2015/150731/ncomms8859/fig_tab/ncomms8859_F3.html open or closed conformation] differentiating between the receptor's active and inactive states. The active, or open conformation, is characterized by an intracellular outward movement of helicies V and VI (breaking hydrogen bonds between <scene name='72/721538/Arg173-ser350_h_bond/1'>Arg173-Ser350</scene> and <scene name='72/721538/Arg173-ser350_h_bond/2'>Glu245-Thr351</scene>) ''citation needed'' and an extracellular rotation of the ECD until it is almost perpendicular to the membrane surface ''citation needed''. While the stalk region of Helix I helps to facilitate the motion of the ECD, intracellular G-protein coupling and extracellular glucagon binding stabilized this active state. In the abscence of glucagon, however, the GCGR adopts a closed conformation in which all three of the extracellular loops of the 7tm (ECL1, ECL2, and ECL3) can interact with the ECD ''citation needed''. In this closed state, the ECD covers the extracellular surface of the 7tm. To transition between states, the ECD rotates and moves down towards the 7tm domain. This transition mechanism is consistent with the "two-domain" binding mechanism of class B GCPRs in which (1) the C-terminus of the ligand first binds to the ECD allowing (2) the N-terminus of the ligand to interact with the 7tm and activate the protein ''citation needed''. | + | Research has shown that class B GCPRs exist in either an [http://www.nature.com/ncomms/2015/150731/ncomms8859/fig_tab/ncomms8859_F3.html open or closed conformation] differentiating between the receptor's active and inactive states. The active, or open conformation, is characterized by an intracellular outward movement of <scene name='72/721538/Helix_v_and_vi/1'>helicies V and VI</scene> (breaking hydrogen bonds between <scene name='72/721538/Arg173-ser350_h_bond/1'>Arg173-Ser350</scene> and <scene name='72/721538/Arg173-ser350_h_bond/2'>Glu245-Thr351</scene>) ''citation needed'' and an extracellular rotation of the ECD until it is almost perpendicular to the membrane surface ''citation needed''. While the stalk region of Helix I helps to facilitate the motion of the ECD, intracellular G-protein coupling and extracellular glucagon binding stabilized this active state. In the abscence of glucagon, however, the GCGR adopts a closed conformation in which all three of the extracellular loops of the 7tm (ECL1, ECL2, and ECL3) can interact with the ECD ''citation needed''. In this closed state, the ECD covers the extracellular surface of the 7tm. To transition between states, the ECD rotates and moves down towards the 7tm domain. This transition mechanism is consistent with the "two-domain" binding mechanism of class B GCPRs in which (1) the C-terminus of the ligand first binds to the ECD allowing (2) the N-terminus of the ligand to interact with the 7tm and activate the protein ''citation needed''. |
Revision as of 06:11, 19 April 2016
| This Sandbox is Reserved from Jan 11 through August 12, 2016 for use in the course CH462 Central Metabolism taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1160 through Sandbox Reserved 1184. |
To get started:
More help: Help:Editing |
Class B Human Glucagon G-Protein Coupled Receptor
References
- ↑ Lotfy M, Kalasz H, Szalai G, Singh J, Adeghate E. Recent Progress in the Use of Glucagon and Glucagon Receptor Antago-nists in the Treatment of Diabetes Mellitus. Open Med Chem J. 2014 Dec 31;8:28-35. doi: 10.2174/1874104501408010028., eCollection 2014. PMID:25674162 doi:http://dx.doi.org/10.2174/1874104501408010028
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, Liu W, Lau J, Cherezov V, Katritch V, Wang MW, Stevens RC. Structure of the human glucagon class B G-protein-coupled receptor. Nature. 2013 Jul 25;499(7459):444-9. doi: 10.1038/nature12393. Epub 2013 Jul 17. PMID:23863937 doi:10.1038/nature12393
- ↑ Koth CM, Murray JM, Mukund S, Madjidi A, Minn A, Clarke HJ, Wong T, Chiang V, Luis E, Estevez A, Rondon J, Zhang Y, Hotzel I, Allan BB. Molecular basis for negative regulation of the glucagon receptor. Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14393-8. Epub 2012 Aug 20. PMID:22908259 doi:http://dx.doi.org/10.1073/pnas.1206734109
- ↑ Mukund S, Shang Y, Clarke HJ, Madjidi A, Corn JE, Kates L, Kolumam G, Chiang V, Luis E, Murray J, Zhang Y, Hotzel I, Koth CM, Allan BB. Inhibitory mechanism of an allosteric antibody targeting the glucagon receptor. J Biol Chem. 2013 Nov 4. PMID:24189067 doi:http://dx.doi.org/10.1074/jbc.M113.496984