We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1160
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Discovery == | == Discovery == | ||
The mGlu family of receptors was the first of the Class C [[GPCR]] to be extensively studied<ref name="Wu" />. The first regions of the protein crystallized and studied were the Venus Fly Trap domain and the cysteine -rich domain on the extracellular region of the receptor<ref name="Dore" />.The Venus Flytrap domain is a large extracellular domain that will selectively bind to glutamate<ref name="Wu" />. The CRD is a somewhat smaller domain composed of many ß sheets and cysteine residues <ref name="Wu" />. The CRD acts as a signal mediator between the Venus Flytrap domain and the TMD of mGlu5, by linking to each domain with disulfide bonds<ref name="Wu" />. The hydrophobic nature and flexibility of the mGlu5 TMD made it difficult to crystallize. | The mGlu family of receptors was the first of the Class C [[GPCR]] to be extensively studied<ref name="Wu" />. The first regions of the protein crystallized and studied were the Venus Fly Trap domain and the cysteine -rich domain on the extracellular region of the receptor<ref name="Dore" />.The Venus Flytrap domain is a large extracellular domain that will selectively bind to glutamate<ref name="Wu" />. The CRD is a somewhat smaller domain composed of many ß sheets and cysteine residues <ref name="Wu" />. The CRD acts as a signal mediator between the Venus Flytrap domain and the TMD of mGlu5, by linking to each domain with disulfide bonds<ref name="Wu" />. The hydrophobic nature and flexibility of the mGlu5 TMD made it difficult to crystallize. | ||
| - | Recently, the human metabotropic glutamate receptor 5 transmembrane domain (TMD) was crystallized and a structure elucidated<ref name="Dore" />. Several modifications were made to the TMD for successful crystallization. The protein was thermostabilized and flexible domains were removed<ref name="Dore" />. In total residues 2-568 and 837-1153 were excised from the structure. | + | |
| + | Recently, the human metabotropic glutamate receptor 5 transmembrane domain (TMD) was crystallized and a structure elucidated<ref name="Dore" />. Several modifications were made to the TMD for successful crystallization. The protein was thermostabilized and flexible domains were removed<ref name="Dore" />. In total residues 2-568 and 837-1153 were excised from the structure. These flexible domains allow mGlu5 to bind to its GPCR <ref name="Dore" />. The structure of the alpha helices are shown in <font color='darkgreen'>'''green'''</font>, and the negative allosteric modulator mavoglurant shown in <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>. Also in <font color='orange'><b>orange</b></font>, a T4 -<scene name='72/721531/Protien_lys/3'>Lysozyme</scene> was inserted into intracellular loop 2 to add stability<ref name="Dore" />. | ||
== Structure== | == Structure== | ||
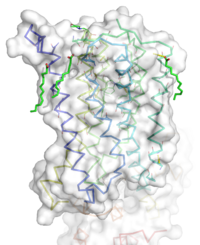
| - | [[Image:STR.png|200 px|left|thumb|'''Figure 1''': Overall Structure of the mGlu5 TMD. The polar heads on the | + | [[Image:STR.png|200 px|left|thumb|'''Figure 1''': Overall Structure of the mGlu5 TMD. The polar heads on the Oleic acids orient the image with the top oriented extracellularly, the middle portion inserted into the membrane, and the lower portion oriented intracellularly. The white exterior represents the surface of the protien, and the multicolored lines interior to the surface represent the backbones 7 transmembrane alpha helices. ]] |
=== Overview === | === Overview === | ||
| - | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/4'> alpha helices</scene> that spans the membrane. The protein was crystallized with <scene name='72/721531/Protien_clean_sce/6'>Oleic acid</scene> shown in <font color='red'>'''red'''</font>. On the top portion of the protein several critical extracellular loops connect the TMD to the CRD. The binding pocket can be found | + | The mGlu5 TMD contains 7 <scene name='72/721531/Protien_7_helices/4'> alpha helices</scene> that spans the membrane. The protein was crystallized with <scene name='72/721531/Protien_clean_sce/6'>Oleic acid</scene> shown in <font color='red'>'''red'''</font>. On the top portion of the protein several critical extracellular loops connect the TMD to the CRD. The binding pocket can be found deep in the interior of the protein, and is mainly comprised of hydrophobic amino acids with more polar amino acids found in the upper and lower portions of the binding site<ref name="Dore" />. Inserted into the biding pocket is the negative allosteric modulator [http://www.en.wikipedia.org/wiki/Mavoglurant mavoglurant]. The TMD is in an inactive conformation, since mavoglurant is bound<ref name="Dore" />. Also, the deletion of the flexible domains leaves the mGlu5 receptor unable to bind to its [[GPCR]]<ref name="Dore" />. The inactive state is maintained by multiple ionic locks whose positions determine the active versus inactive conformation. |
When mGlu5 is in the active conformation, signaling begins with glutamate binding to the Venus flytrap domain. The signal is transduced across the cystine-rich domain to the TMD<ref name="Niswender" />. Next, the dimerization of the TMD occurs. This activates the Gq/11 pathway, which activates phospholipase Cβ<ref name="Niswender" />. The active [http://www.proteopedia.org/wiki/index.php/2zkm phospholipase Cβ] hydrolyzes phosphotinositides and generates [https://pubchem.ncbi.nlm.nih.gov/compound/439456#section=Top inositol 1,4,5-trisphosphate] and [http://www.sivabio.50webs.com/ip3.htm diacyl-glycerol]<ref name="Woodcock" />. This results in calcium mobilization and activation of protein kinase C ([[PKC]])<ref name="Niswender" />. Calcium is a neurotransmitter, and relatively low concentrations of calcium can cause a large response across the neuronal synapse<ref name="Niswender" />. In addition to calcium stimulating an exitory response in nerve cells, PKC can be activated for regulatory purposes with the influx of calcium. A serine on PKC can become phosphorylated which leaves it unable to bind to G beta-gamma (Gßγ) protein complex<ref name="Niswender" />. Unbound Gßγ protein can then inhibit voltage-sensitive calcium channels to reduce calcium influx and provide feedback inhibition to the glutamate signaling pathway. <ref name="Niswender" />. | When mGlu5 is in the active conformation, signaling begins with glutamate binding to the Venus flytrap domain. The signal is transduced across the cystine-rich domain to the TMD<ref name="Niswender" />. Next, the dimerization of the TMD occurs. This activates the Gq/11 pathway, which activates phospholipase Cβ<ref name="Niswender" />. The active [http://www.proteopedia.org/wiki/index.php/2zkm phospholipase Cβ] hydrolyzes phosphotinositides and generates [https://pubchem.ncbi.nlm.nih.gov/compound/439456#section=Top inositol 1,4,5-trisphosphate] and [http://www.sivabio.50webs.com/ip3.htm diacyl-glycerol]<ref name="Woodcock" />. This results in calcium mobilization and activation of protein kinase C ([[PKC]])<ref name="Niswender" />. Calcium is a neurotransmitter, and relatively low concentrations of calcium can cause a large response across the neuronal synapse<ref name="Niswender" />. In addition to calcium stimulating an exitory response in nerve cells, PKC can be activated for regulatory purposes with the influx of calcium. A serine on PKC can become phosphorylated which leaves it unable to bind to G beta-gamma (Gßγ) protein complex<ref name="Niswender" />. Unbound Gßγ protein can then inhibit voltage-sensitive calcium channels to reduce calcium influx and provide feedback inhibition to the glutamate signaling pathway. <ref name="Niswender" />. | ||
Revision as of 10:51, 19 April 2016
Human metabotropic glutamate receptor 5 transmembrane domain
| |||||||||||