This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Brittany Stankavich/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
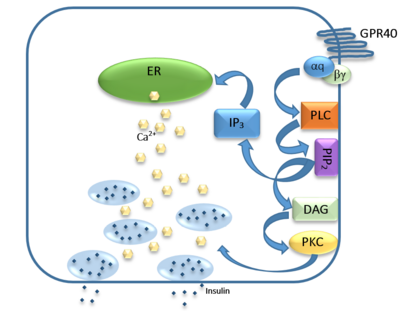
GPR40’s natural substrate are FFAs in which a free [https://en.wikipedia.org/wiki/Carboxylic_acid carboxyl group] is required to bind. However, GPR40 can be activated by a wide variety of fatty acids with chain lengths ranging from [https://en.wikipedia.org/wiki/Saturated_fat saturated fatty acids] with 8 carbons to 23 carbons. In addition, various [https://en.wikipedia.org/wiki/Monounsaturated_fat mono] (i.e. [https://en.wikipedia.org/wiki/Palmitoleic_acid palmitoleic] (C16:1) and [https://en.wikipedia.org/wiki/Oleic_acid oleic] (C18:1) acids) and [https://en.wikipedia.org/wiki/Polyunsaturated_fatty_acid poly-unsaturated fatty acids] (i.e.[https://en.wikipedia.org/wiki/Linoleic_acid linoleic] (C18:2) and [https://en.wikipedia.org/wiki/List_of_unsaturated_fatty_acids#Eicosatrienoic_acid eicosatrienoic] (C20:3) acids) can activate GPR40 <ref name= "Morg"/>. The agonists potency varies according to the carbon-chain length however. The activity of GPR40 increases when the chain is increased from C6 to C15 but then decreased when the chain was extended beyond C15. One explanation for this is that as [https://en.wikipedia.org/wiki/Alkyl alkyl] chain increased, so did the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] interactions with the protein within the binding pocket. However, for FFAs with carbon chains longer than C15, the molecular size is too large for the binding pocket. This causes the alkyl chain to extend beyond the binding pocket and destabilize the binding <ref name="Ren">PMID:26974599</ref>. | GPR40’s natural substrate are FFAs in which a free [https://en.wikipedia.org/wiki/Carboxylic_acid carboxyl group] is required to bind. However, GPR40 can be activated by a wide variety of fatty acids with chain lengths ranging from [https://en.wikipedia.org/wiki/Saturated_fat saturated fatty acids] with 8 carbons to 23 carbons. In addition, various [https://en.wikipedia.org/wiki/Monounsaturated_fat mono] (i.e. [https://en.wikipedia.org/wiki/Palmitoleic_acid palmitoleic] (C16:1) and [https://en.wikipedia.org/wiki/Oleic_acid oleic] (C18:1) acids) and [https://en.wikipedia.org/wiki/Polyunsaturated_fatty_acid poly-unsaturated fatty acids] (i.e.[https://en.wikipedia.org/wiki/Linoleic_acid linoleic] (C18:2) and [https://en.wikipedia.org/wiki/List_of_unsaturated_fatty_acids#Eicosatrienoic_acid eicosatrienoic] (C20:3) acids) can activate GPR40 <ref name= "Morg"/>. The agonists potency varies according to the carbon-chain length however. The activity of GPR40 increases when the chain is increased from C6 to C15 but then decreased when the chain was extended beyond C15. One explanation for this is that as [https://en.wikipedia.org/wiki/Alkyl alkyl] chain increased, so did the [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] interactions with the protein within the binding pocket. However, for FFAs with carbon chains longer than C15, the molecular size is too large for the binding pocket. This causes the alkyl chain to extend beyond the binding pocket and destabilize the binding <ref name="Ren">PMID:26974599</ref>. | ||
| - | FFAs bind to hGPR40 by coordinating its free carboxyl group to three amino acids, <scene name='72/727085/Ffa_binding/1'>Arg183, Tyr2240, and Arg258</scene>, which are located close to the <scene name='72/727085/Hgpr40_transmane_active/1'>extracellular domain</scene> of hGPR40 on TM5, 6 and 7. Because of the close proximity of these residues to the extracellular domain and the dominantly hydrophobic nature of FFA’s, it is | + | FFAs bind to hGPR40 by coordinating its free carboxyl group to three amino acids, <scene name='72/727085/Ffa_binding/1'>Arg183, Tyr2240, and Arg258</scene>, which are located close to the <scene name='72/727085/Hgpr40_transmane_active/1'>extracellular domain</scene> of hGPR40 on TM5, 6 and 7. Because of the close proximity of these residues to the extracellular domain and the dominantly hydrophobic nature of FFA’s, it is likely that ligand binding occurs close to the plane of the membrane <ref name="Morg">PMID:19660440</ref>. |
=== [http://proteopedia.org/wiki/index.php/Image:Tak_875.png TAK-875] === | === [http://proteopedia.org/wiki/index.php/Image:Tak_875.png TAK-875] === | ||
| - | <scene name='72/727085/Hgpr40_begin/3'> | + | <scene name='72/727085/Hgpr40_begin/3'>Tak-875</scene> is a [https://en.wikipedia.org/wiki/Partial_agonist partial agonist] of GPR40 and tested for the treatment of type 2 diabetes. The binding of TAK-875 to hGPR40 occurs by the ligand entering the binding site through the [https://en.wikipedia.org/wiki/Cell_membrane membrane bilayer]. This membrane insertion is performed via a method similar to ligand binding to [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate_receptor sphingosine 1-phosphate receptor 1], retinal loading of [http://proteopedia.org/wiki/index.php/4j4q GPCR opsin], and the entry of anandamide in [https://en.wikipedia.org/wiki/Cannabinoid_receptor cannabinoid receptors], in which <scene name='72/727085/Hgpr40_a/6'>extracellular loops</scene> block the binding from the extracellular matrix <ref>PMID:22344443</ref>. The binding mechanism through the bilayer may be selectively favoring the free fatty acid because of the [https://en.wikipedia.org/wiki/Chemical_polarity#Nonpolar_molecules non-polar] regions of the ligand. |
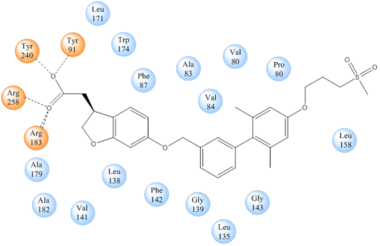
TAK-875 binds to a non-canonical site created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. A disulfide bond is created between Cys79 and Cys170 which increases the rigidity of the ECL2 and allows it to stay in place and function as a cap to the binding site. The carboxylate of TAK-875 is buried within a very hydrophobic region where participates in a complex <scene name='72/727085/Hgpr40_binding_relay/6'>charge network</scene> involving Glu172, Ser187, Asn241, and Asn 244 within the binding pocket of hGPR40 forming ionic and polar interactions with Arg183, Arg258, Tyr91, and Tyr240. | TAK-875 binds to a non-canonical site created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. A disulfide bond is created between Cys79 and Cys170 which increases the rigidity of the ECL2 and allows it to stay in place and function as a cap to the binding site. The carboxylate of TAK-875 is buried within a very hydrophobic region where participates in a complex <scene name='72/727085/Hgpr40_binding_relay/6'>charge network</scene> involving Glu172, Ser187, Asn241, and Asn 244 within the binding pocket of hGPR40 forming ionic and polar interactions with Arg183, Arg258, Tyr91, and Tyr240. | ||
Revision as of 11:30, 22 April 2016
- User:Brittany Stankavich/Sandbox 1
hGPR40 Homo sapiens
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, Lane W, Ivetac A, Aertgeerts K, Nguyen J, Jennings A, Okada K. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature. 2014 Jul 20. doi: 10.1038/nature13494. PMID:25043059 doi:http://dx.doi.org/10.1038/nature13494
- ↑ Ren XM, Cao LY, Zhang J, Qin WP, Yang Y, Wan B, Guo LH. Investigation of the Binding Interaction of Fatty Acids with Human G Protein-Coupled Receptor 40 Using a Site-Specific Fluorescence Probe by Flow Cytometry. Biochemistry. 2016 Mar 17. PMID:26974599 doi:http://dx.doi.org/10.1021/acs.biochem.6b00079
- ↑ 3.0 3.1 3.2 3.3 3.4 Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009 Sep;89(3-4):82-8. doi:, 10.1016/j.prostaglandins.2009.05.003. Epub 2009 May 19. PMID:19460454 doi:http://dx.doi.org/10.1016/j.prostaglandins.2009.05.003
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Morgan NG, Dhayal S. G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem Pharmacol. 2009 Dec 15;78(12):1419-27. doi: 10.1016/j.bcp.2009.07.020., Epub 2009 Aug 4. PMID:19660440 doi:http://dx.doi.org/10.1016/j.bcp.2009.07.020
- ↑ Ren XM, Cao LY, Zhang J, Qin WP, Yang Y, Wan B, Guo LH. Investigation of the Binding Interaction of Fatty Acids with Human G Protein-Coupled Receptor 40 Using a Site-Specific Fluorescence Probe by Flow Cytometry. Biochemistry. 2016 Mar 17. PMID:26974599 doi:http://dx.doi.org/10.1021/acs.biochem.6b00079
- ↑ Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012 Feb 17;335(6070):851-5. PMID:22344443 doi:10.1126/science.1215904
- ↑ Li X, Zhong K, Guo Z, Zhong D, Chen X. Fasiglifam (TAK-875) Inhibits Hepatobiliary Transporters: A Possible Factor Contributing to Fasiglifam-Induced Liver Injury. Drug Metab Dispos. 2015 Nov;43(11):1751-9. doi: 10.1124/dmd.115.064121. Epub 2015, Aug 14. PMID:26276582 doi:http://dx.doi.org/10.1124/dmd.115.064121
- ↑ 8.0 8.1 Takano R, Yoshida M, Inoue M, Honda T, Nakashima R, Matsumoto K, Yano T, Ogata T, Watanabe N, Hirouchi M, Yoneyama T, Ito S, Toda N. Discovery of DS-1558: A Potent and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2015 Jan 13;6(3):266-70. doi: 10.1021/ml500391n. eCollection, 2015 Mar 12. PMID:25815144 doi:http://dx.doi.org/10.1021/ml500391n