We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1180
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | =Glucagon G protein coupled receptor= | + | =Glucagon G protein-coupled receptor= |
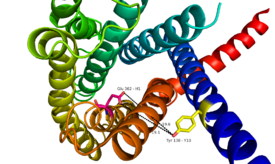
<StructureSection load='4L6R' size='420' side='right' caption='7TM structure of human class B GPCR 4L6R', [[Resolution|resolution]] 1.80Å' scene=''> | <StructureSection load='4L6R' size='420' side='right' caption='7TM structure of human class B GPCR 4L6R', [[Resolution|resolution]] 1.80Å' scene=''> | ||
| Line 10: | Line 10: | ||
| - | ==Glucagon Receptor== | + | ==Glucagon Receptor (GCGR)== |
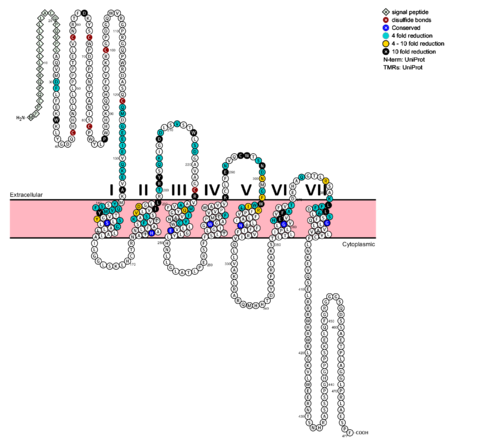
The glucagon class B GPCR (GCGR) is involved in glucose homeostasis through the binding of the signal peptide glucagon. See also [http://sbkb.org/fs/glucagon-receptor PSI Structural Biology Database] Glucagon is released from pancreatic α-cells when blood glucose levels fall after a period of fasting or several hours following intake of dietary carbohydrates.<ref name = 'Lehninger'/> Once the peptide hormone is released, it binds to GCGR, a 485 amino acid protein found in the liver, kidney, intestinal smooth muscle, brain, and adipose tissues. <ref name= "Yang 2015">DOI 10.1038/aps.2015.78</ref> Upon binding, signaling is initiated to heterotrimeric G-proteins containing Gαs. <ref name= "Ahren 2009">DOI 10.1038/nrd2782</ref> GCGR can regulate additional signal pathways, including G-proteins of the Gαi family through the adoption of differing receptor conformations. <ref name= "Xu 2009">DOI 10.3109/10799890903295150</ref> | The glucagon class B GPCR (GCGR) is involved in glucose homeostasis through the binding of the signal peptide glucagon. See also [http://sbkb.org/fs/glucagon-receptor PSI Structural Biology Database] Glucagon is released from pancreatic α-cells when blood glucose levels fall after a period of fasting or several hours following intake of dietary carbohydrates.<ref name = 'Lehninger'/> Once the peptide hormone is released, it binds to GCGR, a 485 amino acid protein found in the liver, kidney, intestinal smooth muscle, brain, and adipose tissues. <ref name= "Yang 2015">DOI 10.1038/aps.2015.78</ref> Upon binding, signaling is initiated to heterotrimeric G-proteins containing Gαs. <ref name= "Ahren 2009">DOI 10.1038/nrd2782</ref> GCGR can regulate additional signal pathways, including G-proteins of the Gαi family through the adoption of differing receptor conformations. <ref name= "Xu 2009">DOI 10.3109/10799890903295150</ref> | ||
| Line 20: | Line 20: | ||
| - | ==Structure== | + | ===Structure=== |
The class B GPCRs, including GCGR, are different from other GPCRs in several ways. The first is that class B GPCRs contain a protrusion known as a 'stalk,' a three α-helical turn elongation of the N-terminus that protrudes past the extracellular (EC) membrane. Structural integrity of this domain in GCGR is <scene name='72/721552/Ligand_binding_interactions/1'>essential to ligand binding affinity</scene> in that A135P mutations effect stalk stability through the removal of an important salt bridge between Glu133-Lys136. | The class B GPCRs, including GCGR, are different from other GPCRs in several ways. The first is that class B GPCRs contain a protrusion known as a 'stalk,' a three α-helical turn elongation of the N-terminus that protrudes past the extracellular (EC) membrane. Structural integrity of this domain in GCGR is <scene name='72/721552/Ligand_binding_interactions/1'>essential to ligand binding affinity</scene> in that A135P mutations effect stalk stability through the removal of an important salt bridge between Glu133-Lys136. | ||
| Line 35: | Line 35: | ||
| - | ==Glucagon Binding= | + | ===Glucagon Binding=== |
| Line 43: | Line 43: | ||
GPCR activity is regularly quantified by ligand binding affinity, potency, efficacy, and kinetics. These measurement are used to measure drug ligand interactions in vivo. Recently, GPCRs have been crystallized and catalogued, which tend to include a need to stabilize the receptor, emphasizing the instability of the G coupled protein receptor. Zhang et. al. imply the importance of receptor folding in the cell membrane, in the human class B GPCR the 7TM portion, for receptor stability and function. <ref>DOI 10.1016/j.tibs.2014.12.005</ref> | GPCR activity is regularly quantified by ligand binding affinity, potency, efficacy, and kinetics. These measurement are used to measure drug ligand interactions in vivo. Recently, GPCRs have been crystallized and catalogued, which tend to include a need to stabilize the receptor, emphasizing the instability of the G coupled protein receptor. Zhang et. al. imply the importance of receptor folding in the cell membrane, in the human class B GPCR the 7TM portion, for receptor stability and function. <ref>DOI 10.1016/j.tibs.2014.12.005</ref> | ||
| - | |||
| - | ==Structural Considerations== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | It has been discovered that the large, soluble N-terminal extracellular domains (ECD) of GCGR are primary in ligand selectivity with the deep, ligand pocket (Fig. 7) of the TMD providing secondary recognition. <ref name= "Yang 2015"/> | ||
| - | |||
| - | Because of the difficulty of stabilizing and crystallizing Class B TMDs, very little is known about the conformational changes that transduce cell signals endogenously. It is known that GCGR can regulate additional signal pathways including G-proteins of the Gαi family through the adoption of differing receptor conformations. Research is ongoing. <ref name= "Xu 2009"/> | ||
| - | |||
| - | GCGR generates downstream signals predominantly through the increase of intracellular cAMP, however there are other pathways being uncovered that are the result of GCGR adopting multiple, active conformations. Researchers are currently investigating how receptor activity-modifying proteins (RAMPs) interact with the ligand and GCGR in which the signaling bias of the receptor is altered. <ref>DOI 10.1074/jbc.M114.624601</ref> | ||
| - | |||
| - | GPCR activity is regularly quantified by ligand binding affinity, potency, efficacy, and kinetics. These measurement are used to measure drug ligand interactions in vivo. Recently, GPCRs have been crystallized and catalogued, which tend to include a need to stabilize the receptor, emphasizing the instability of the G coupled protein receptor. Zhang et. al. imply the importance of receptor folding in the cell membrane, in the human class B GPCR the 7TM portion, for receptor stability and function. <ref>DOI 10.1016/j.tibs.2014.12.005</ref> | ||
| - | |||
| - | |||
Revision as of 20:44, 21 April 2016
Glucagon G protein-coupled receptor
| |||||||||||