Sandbox Reserved 1180
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
===Glucagon Binding=== | ===Glucagon Binding=== | ||
| + | |||
| + | The large, soluble N-terminal extracellular domains (ECD) of GCGR provide initial ligand selectivity with the deep, ligand pocket (Fig. 2) of the TMD providing secondary recognition.<ref name= "Yang 2015"/> | ||
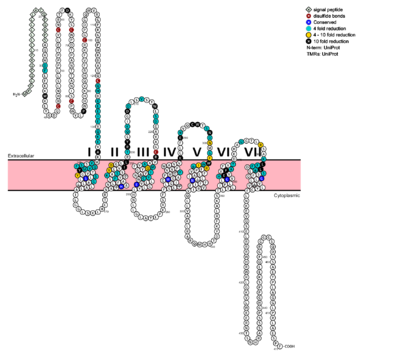
[[Image:Protter GLR HUMAN.png |400 px|left|thumb|Fig. 1: Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.<ref name= "Siu 2013"/>]] | [[Image:Protter GLR HUMAN.png |400 px|left|thumb|Fig. 1: Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.<ref name= "Siu 2013"/>]] | ||
In a comprehensive mutagenesis and glucagon-binding study, a total of 129 mutations of GCGR were tested. 41 of these covering 28 different locations in the GCGR TMD were found to have at least a fourfold decrease in glucagon binding affinity<ref name= "Siu 2013"/>. (see Fig. 1) It is the face of the central cavity that harbors the majority of the residues which play an important role in glucagon binding.<ref name= "Siu 2013"/> The binding site was shown to be a dynamic area traveling from the middle of the stalk region (Tyr 138) to deep within the 7TM core (Glu 362), encompassing positions along ECL1, ECL2 and ECL3 and helices I, II, III, V, VI and VII. | In a comprehensive mutagenesis and glucagon-binding study, a total of 129 mutations of GCGR were tested. 41 of these covering 28 different locations in the GCGR TMD were found to have at least a fourfold decrease in glucagon binding affinity<ref name= "Siu 2013"/>. (see Fig. 1) It is the face of the central cavity that harbors the majority of the residues which play an important role in glucagon binding.<ref name= "Siu 2013"/> The binding site was shown to be a dynamic area traveling from the middle of the stalk region (Tyr 138) to deep within the 7TM core (Glu 362), encompassing positions along ECL1, ECL2 and ECL3 and helices I, II, III, V, VI and VII. | ||
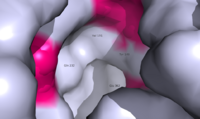
| + | [[Image:Movie Frame 6.png |200 px|center|thumb|Fig. 2: <scene name='72/721552/Glucagon_binding/3'>Deep, central cavity</scene> functioning as anchoring site for glucagon's n-terminal residues.]][[Image:Glucagon with Q3 and N-terminus.png |200 px|right|thumb|Fig. 3: Surface visualization of glucagon visualizing the three dimensional shape of the N-terminal tail that interacts with the binding site of GCGR central cavity.]] | ||
| - | Mutagenesis and photo cross-linking studies determined essential, conserved residues in glucagon and have been <scene name='72/727091/Glucagon_important_residues/2'>labeled and colored</scene> in red.<ref name= "Siu 2013"/> Glucagon residues His 1, Gln 3, Phe 6, and Tyr 10 are critical to successful binding interaction with the GCGR while others are important for structural rigidity. The n-terminal random coil of glucagon (Fig. 2) leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TM (Fig. 3). There is a <scene name='72/721552/Glucagon_binding_zoomed_in/1'>narrow neck</scene> to the entrance of the cavity, providing a firm anchor during peptide docking. | ||
| - | [[Image:Glucagon with Q3 and N-terminus.png |200 px|right|thumb|Fig. 2: Surface visualization of glucagon visualizing the three dimensional shape of the N-terminal tail that interacts with the binding site of GCGR central cavity.]] | ||
| - | [[Image:Movie Frame 6.png |200 px|right|thumb|Fig. 3: Ballooned pocket functioning as anchoring site for <scene name='72/721552/Glucagon_binding/3'>glucagon's n-terminal residues</scene>.]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | It has been discovered that the large, soluble N-terminal extracellular domains (ECD) of GCGR are primary in ligand selectivity with the deep, ligand pocket (Fig. 7) of the TMD providing secondary recognition. <ref name= "Yang 2015"/> | ||
| - | |||
| - | |||
| - | |||
| - | GPCR activity is regularly quantified by ligand binding affinity, potency, efficacy, and kinetics. These measurement are used to measure drug ligand interactions in vivo. Recently, GPCRs have been crystallized and catalogued, which tend to include a need to stabilize the receptor, emphasizing the instability of the G coupled protein receptor. Zhang et. al. imply the importance of receptor folding in the cell membrane, in the human class B GPCR the 7TM portion, for receptor stability and function. <ref>DOI 10.1016/j.tibs.2014.12.005</ref> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | Through mutagenesis and photo-crosslinking studies, several residues deep within the central cavity of the GCGR 7TMD were discovered neighboring Glu362, which is approximately 19 angstroms from the base of the EC stalk and the location of Tyr138. (Fig. 8) | ||
| - | |||
| - | |||
| - | Four essential residues exist deep within the central cavity which all play strong roles in ligand binding affinity. (Fig. 9) | ||
| - | |||
| - | |||
| - | |||
| - | A narrow entry gives way to a large, anchoring site for residues 1-4 of glucagon. (Fig. 10) | ||
| - | |||
| - | Essential to glucagon's binding, a long, N-terminal tail winds to a clump of 4 residues, culminating in bulge that fits into the central, anchoring site of the 7TMD. (Fig. 11) | ||
| + | Mutagenesis and photo cross-linking studies determined essential, conserved residues in glucagon and have been <scene name='72/727091/Glucagon_important_residues/2'>labeled and colored</scene> in red.<ref name= "Siu 2013"/> Glucagon residues His 1, Gln 3, Phe 6, and Tyr 10 are critical to successful binding interaction with the GCGR while others are important for structural rigidity. The n-terminus of glucagon (Fig. 3) leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TMD (Fig. 2) where four residues reside that play strong roles in ligand binding affinity. There is a <scene name='72/721552/Glucagon_binding_zoomed_in/1'>narrow neck</scene> to the entrance of the cavity, providing a firm anchor during peptide docking. | ||
Revision as of 22:59, 21 April 2016
Glucagon G protein-coupled receptor
| |||||||||||