Sandbox HEC
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
Human erythrocyte catalase is used to protect hemoglobin by removing hydrogen peroxide generated from erythrocytes. Human catalase is a heme-containing enzyme whose primary function is to break down hydrogen peroxide into two molecules of water and one molecule of oxygen. Human catalase plays a major part in the defense against oxidative damage and inactivation of hemoglobin by removing the hydrogen peroxide formed by human erythrocytes <ref name="putnam">PMID:10656833</ref> . Hydrogen peroxide is a byproduct of normal cellular respiration, but is toxic at high concentrations. If catalase does not break down hydrogen peroxide, it gets converted into a reactive oxygen species and can damage DNA, proteins, and cell membranes <ref name="goth">PMID:15771551</ref>. Human catalase enzyme has been noted as an important factor in prevention of apoptosis and stimulation of tumors. During a normal catalytic cycle hydrogen peroxide is the source of both oxidative and reductive potential. NADPH has been known to also bind to human catalase, however, it does not serve as an oxidizing or reducing agent, but protects the catalase from being inactivated by hydrogen peroxide <ref name="putnam" />. | Human erythrocyte catalase is used to protect hemoglobin by removing hydrogen peroxide generated from erythrocytes. Human catalase is a heme-containing enzyme whose primary function is to break down hydrogen peroxide into two molecules of water and one molecule of oxygen. Human catalase plays a major part in the defense against oxidative damage and inactivation of hemoglobin by removing the hydrogen peroxide formed by human erythrocytes <ref name="putnam">PMID:10656833</ref> . Hydrogen peroxide is a byproduct of normal cellular respiration, but is toxic at high concentrations. If catalase does not break down hydrogen peroxide, it gets converted into a reactive oxygen species and can damage DNA, proteins, and cell membranes <ref name="goth">PMID:15771551</ref>. Human catalase enzyme has been noted as an important factor in prevention of apoptosis and stimulation of tumors. During a normal catalytic cycle hydrogen peroxide is the source of both oxidative and reductive potential. NADPH has been known to also bind to human catalase, however, it does not serve as an oxidizing or reducing agent, but protects the catalase from being inactivated by hydrogen peroxide <ref name="putnam" />. | ||
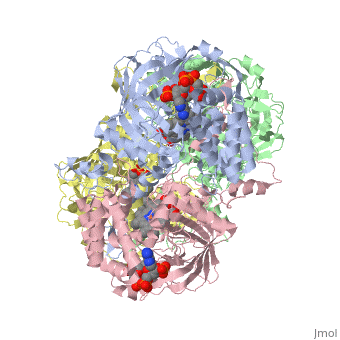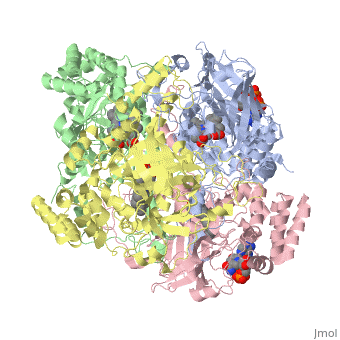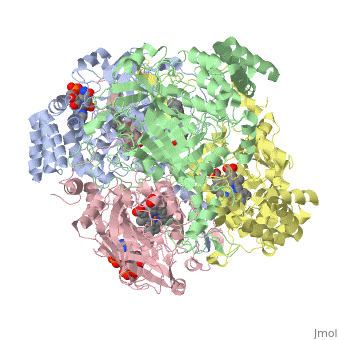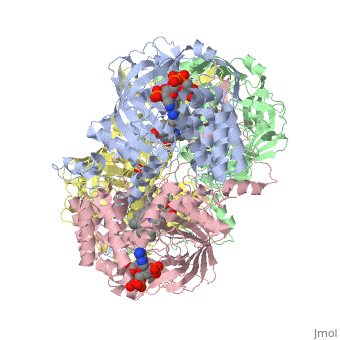
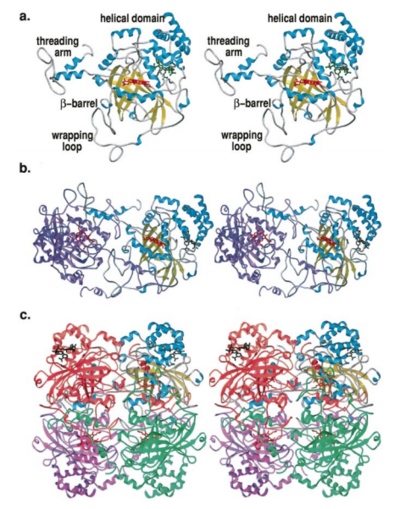
| - | [[Image:Hec.jpg.png | thumb |left|400px|'''Structure of Human Erythrocyte Catalase''' This figure | + | [[Image:Hec.jpg.png | thumb |left|400px|'''Structure of Human Erythrocyte Catalase''' This figure shows an individual subunit of human catalase (a) , an arm-exchanged dimer with a catalase fold where both heme active sites are exposed on one surface (b), and a catalase tetramer with the addition of a second arm exchanged |
dimer where the heme active site is buried within the enzyme. In this figure the beta-barrel domain is colored yellow, the alpha helices are blue, NADPH is dark green, and the active site heme is red. <ref name="putnam" />]] | dimer where the heme active site is buried within the enzyme. In this figure the beta-barrel domain is colored yellow, the alpha helices are blue, NADPH is dark green, and the active site heme is red. <ref name="putnam" />]] | ||
| Line 30: | Line 30: | ||
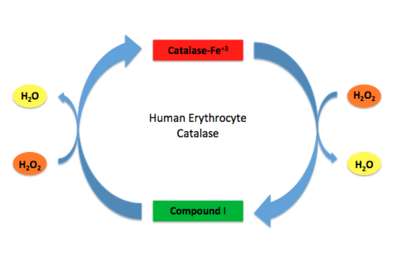
Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, as well as immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body <ref name= Lennicke >PMID:26369938</ref>. Hydrogen peroxide acts to both the oxidizing and reducing agent of the iron. Catalase ultimately functions to break down hydrogen peroxide<ref name="Dash" />. This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water <ref name="Alfonso-Prietro" /><ref name="Diaz" />. The overall reaction results in two single-electron transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, as well as a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers <ref name="Alfonso-Prietro" /><ref name="Diaz" /> . | Stable forms of hydrogen peroxide are beneficial in biological reactions including hypoxia signal transduction, cell proliferation and differentiation regulation, as well as immune response mediation; however, it is toxic at high levels as free hydroxyl ions cannot be catalyzed by the body <ref name= Lennicke >PMID:26369938</ref>. Hydrogen peroxide acts to both the oxidizing and reducing agent of the iron. Catalase ultimately functions to break down hydrogen peroxide<ref name="Dash" />. This is accomplished in a two-step mechanism where the heme is first oxidized by a molecule of hydrogen peroxide to produce Compound I, a high energy oxyferryl cation radical intermediate, as well as a water molecule. Compound I is then immediately reduced by a second hydrogen peroxide molecule to produce a second molecule of water <ref name="Alfonso-Prietro" /><ref name="Diaz" />. The overall reaction results in two single-electron transfers from the iron atom of the heme group and the porphyrin from the oxoferryl radical, as well as a proton transfer from histidine. The mechanism is enthalpically driven by the distal histidine proton transfer as it is more exothermic than the electron transfers <ref name="Alfonso-Prietro" /><ref name="Diaz" /> . | ||
| - | The deeply buried heme group is connected to the protein surface by a primary channel which provides a transport pathway for the hydrogen peroxide substrate <ref name="Diaz" />. The transportation of hydrogen peroxide through the main channel is regulated by electrical dipole interactions between the hydrogen peroxide and the hydrophobic portion of the channel containing negatively charged aspartate and positively charged iron from the heme <ref name="Lennicke" /><ref name="Diaz" />. Additionally, less significant lateral channels allow products to leave the heme pocket<ref name="Diaz | + | The deeply buried heme group is connected to the protein surface by a primary channel which provides a transport pathway for the hydrogen peroxide substrate <ref name="Diaz" />. The transportation of hydrogen peroxide through the main channel is regulated by electrical dipole interactions between the hydrogen peroxide and the hydrophobic portion of the channel containing negatively charged aspartate and positively charged iron from the heme <ref name="Lennicke" /><ref name="Diaz" />. Additionally, less significant lateral channels allow products to leave the heme pocket<ref name="Diaz" />. |
Revision as of 19:40, 27 April 2016
1dgb
| |||||||||||