We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Nos1
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
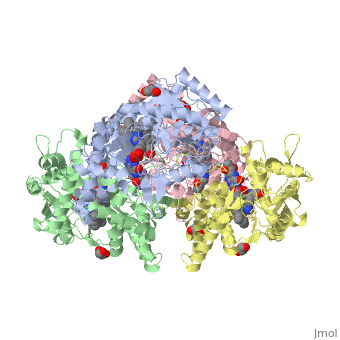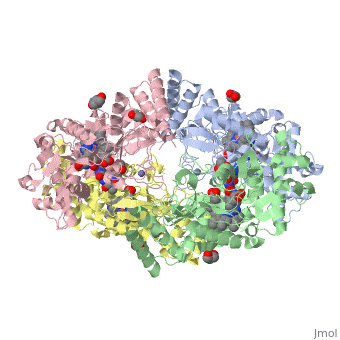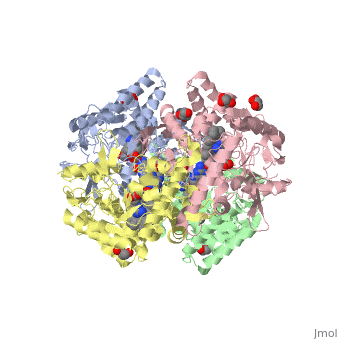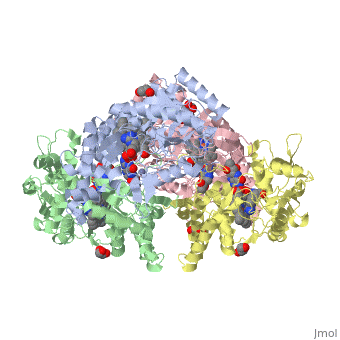
Neuronal nitric oxide synthase exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme. The N-terminal catalytic domain contains a <scene name='72/728110/Heme_active_site/1'>heme active site</scene>, a nearby cofactor site for tetrahydrobiopterin (<scene name='72/728110/H4b/1'>H4b</scene>) and a C-terminal reductase domain that consists of FMN, FAD, and NADPH binding sites.<ref>Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674.</ref> The structure of the human nNOS heme domain contains cysteine and tryptophan. The NADPH provides electrons for catalysis. These electrons are first passed to FAD and FMN and then the heme. This electron flow is monitored by the binding of CaM and Calcium2+ in the linker region between the two heterodimer domains. | Neuronal nitric oxide synthase exists as a homodimer. This homodimer consists of two regions. One region is an N-terminal oxygenase domain and the other is a C-terminal reductase. The N-terminal oxygenase domain is an extended beta sheet cage with binding sites for heme. The N-terminal catalytic domain contains a <scene name='72/728110/Heme_active_site/1'>heme active site</scene>, a nearby cofactor site for tetrahydrobiopterin (<scene name='72/728110/H4b/1'>H4b</scene>) and a C-terminal reductase domain that consists of FMN, FAD, and NADPH binding sites.<ref>Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674.</ref> The structure of the human nNOS heme domain contains cysteine and tryptophan. The NADPH provides electrons for catalysis. These electrons are first passed to FAD and FMN and then the heme. This electron flow is monitored by the binding of CaM and Calcium2+ in the linker region between the two heterodimer domains. | ||
The amino acid composition of asparagine and methionine in rat neuronal NOS are conserved in human nNOS. This amino acid composition influences the binding of inhibitors and makes nNOS inhibitors highly selective. The structure of the human nNOS heme domain contains cysteine and tryptophan. The cysteine subunits of one region pack closely against the backbone of histidine in subunits of another region. Crystal packing interactions most likely contribute to the fully ordered N-termini in the NOS structure. | The amino acid composition of asparagine and methionine in rat neuronal NOS are conserved in human nNOS. This amino acid composition influences the binding of inhibitors and makes nNOS inhibitors highly selective. The structure of the human nNOS heme domain contains cysteine and tryptophan. The cysteine subunits of one region pack closely against the backbone of histidine in subunits of another region. Crystal packing interactions most likely contribute to the fully ordered N-termini in the NOS structure. | ||
| - | There is a flexible surface loop downstream from the <scene name='72/728110/Zinc/1'>Zn2+</scene> binding site. This loop in human nNOS lacks any secondary structure and appears as a random coil. This reflects the flexible nature of nNOS because it can easily adapt to local environment which involves crystal packing. This loop is found near the CaM binding site and can therefore most likely interact with it and affect electron flow. | + | There is a flexible surface loop downstream from the <scene name='72/728110/Zinc/1'>Zn2+</scene> binding site. This loop in human nNOS lacks any secondary structure and appears as a random coil. This reflects the flexible nature of nNOS because it can easily adapt to local environment which involves crystal packing. This loop is found near the CaM binding site and can therefore most likely interact with it and affect electron flow. <ref>Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674.</ref> |
Revision as of 13:49, 28 April 2016
| |||||||||||
References
- ↑ Stuehr DJ. (May 1999) "Mammalian nitric oxide synthases". Biochim. Biophys. Acta 1411 (2–3): 217–30. doi:10.1016/S0005-2728(99)00016-X. PMID 10320659.
- ↑ 2.0 2.1 Knowles RG, Moncada S. (March 1994) "Nitric oxide synthases in mammals". Biochem. J. 298 (2): 249–58. PMC 1137932. PMID 7510950.
- ↑ 3.0 3.1 Ward ME, Toporsian M, Scott JA, et al. (2005) Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. Journal of Clinical Investigation. 115(11):3128-3139. doi:10.1172/JCI20806.
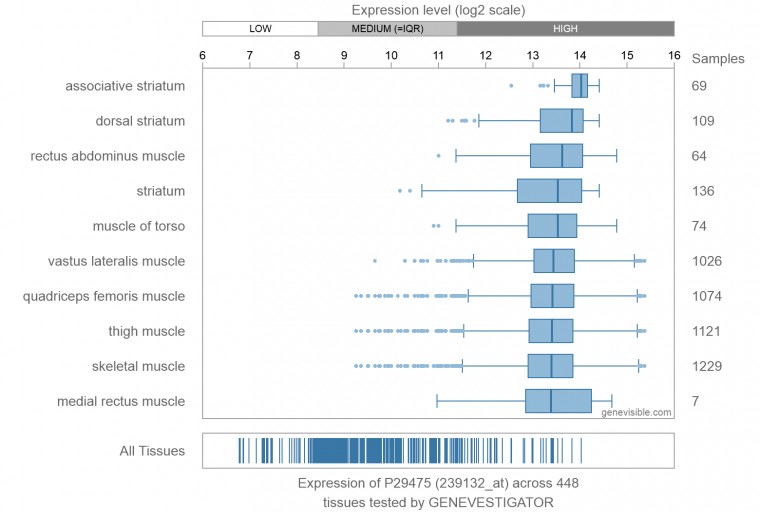
- ↑ Genevisible (ND) 'Expression of P29475' [online] available at https://genevisible.com/tissues/HS/UniProt/P29475
- ↑ Hou, YC; Janczuk, A; Wang, PG. (1999) "Current trends in the development of nitric oxide donors". Current pharmaceutical design 5 (6): 417–41.
- ↑ M Hecker, A Mulsch, R Busse. (1994) Subcellular localization and characterization of neuronal nitric oxide synthase. J. Neurochem., 62, pp. 1524–1529
- ↑ Beigi F, Oskouei BN, Zheng M, Cooke CA, Lamirault G, Hare JM. (2009) Cardiac nitric oxide synthase-1 localization within the cardiomyocyte is accompanied by the adaptor protein, CAPON. Nitric Oxide. 21:226–233. doi: 10.1016/j.niox.2009.09.005.
- ↑ Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, et al. (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 416:337–9. doi: 10.1038/416337a
- ↑ Juliane Kopf, Martin Schecklmann, Tim Hahn, Thomas Dresler, Alica C. Dieler, Martin J. Herrmann, Andreas J. Fallgatter, Andreas Reif. (2011) NOS1 ex1f-VNTR polymorphism influences prefrontal brain oxygenation during a working memory task, NeuroImage, Volume 57, (Issue 4),1617-1623, Ihttp://dx.doi.org/10.1016/
- ↑ Freudenberg, F., Alttoa, A. & Reif, A. (2015) Neuronal nitric oxide synthase (NOS1) and its adaptor (NOS1AP) act as a genetic risk factors for psychiatric. Genes Brain Behav 14, 47–64.
- ↑ 11.0 11.1 Zhang, Y. H., Jin, C. Z., Jang, J. H., & Wang, Y. (2014). Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. The Journal of Physiology, 592(Pt 15), 3189–3200. http://doi.org/10.1113/jphysiol.2013.270306]
- ↑ 12.0 12.1 12.2 12.3 12.4 Shinkai, T., Ohmori, O., Hori, H., and Nakamura, J. (2002) Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Molecular Psychiatry. 7, 560-563. doi:10.1038/sj.mp.4001041
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Galimberti, D., Scarpini, E., Venturelli, E., Strobel, A., Herterich, S., Fenogolio, C., Guidi, I., Scalabrini, D., Cortini, F., Bresolin, N., Lesch, K., and Reif, A. (2008) Association of a NOS1 promoter repeat with Alzheimer’s disease. Neurobiology of Aging. 29, 1359-1365. doi:10.1016/j.neurobiolaging.2007.03.003
- ↑ 14.0 14.1 14.2 14.3 Rife, T., Rasoul, B., Pullen, N., Mitchell, D., Grathwol, K., and Kurth, J. (2009) The effect of a promoter polymorphism on transcription of nitric oxide synthase 1 and its relevance to Parkinson’s disease. Journal of Neuroscience Research. 87, 2319-2325. doi:10.1005/jnr.22045
- ↑ Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674.
- ↑ Li H, Jamal J, Plaza C, et al. Structures of human constitutive nitric oxide synthases. Acta Crystallographica Section D: Biological Crystallography. 2014;70(Pt 10):2667-2674.