This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox WWC7
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
==Mechanism== | ==Mechanism== | ||
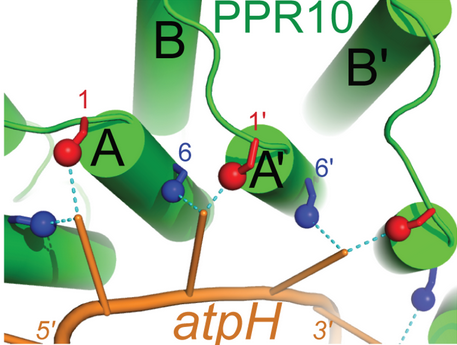
The primary factor in the ability of PPR10 to bind RNA bases in a modular fashion lies in the identities of the residue at position 6 on a repeat and the residue at position 1 on the next repeat. For example, in the structure to the right, <scene name='69/696301/G1binding/1'>threonine residue 178 (blue) forms a hydrogen bond to G1 (green) of ATPH.</scene> Through van der Waals interactions, Val210 and Arg175 (both orange) also contribute to the specific binding of guanine in this example. These residues force G1 into a conformation where it forms a hydrogen bond to Thr178. The example of PPR10 binding G1 of ATPH exemplifies the general rules by which PPR proteins bind specific nucleotides: firstly, a residue at the 6 position of one repeat (Thr178 in the previous example) forms a hydrogen bond with the base. The identity of this residue determines whether the repeat will bind a purine (adenine and guanine) or pyrimidine (cytosine and uracil). It appears that serine and threonine at position 6 are specific for purines, and asparagine at position 6 is specific for pyrimidines.<ref>doi:10.1371/journal.pgen.1002910</ref> Secondly, a residue at position 1 of the next repeat (Val210 in the previous example) completes the specificity of the interaction. Through van der Waals interactions, this residue determines between A/G and C/U. Other amino acids further contribute to this mechanism, but the previously described rules always apply when PPR proteins bind RNA sequences with modularity.<ref name = "engineering">DOI:10.1111/tpj.12377</ref> | The primary factor in the ability of PPR10 to bind RNA bases in a modular fashion lies in the identities of the residue at position 6 on a repeat and the residue at position 1 on the next repeat. For example, in the structure to the right, <scene name='69/696301/G1binding/1'>threonine residue 178 (blue) forms a hydrogen bond to G1 (green) of ATPH.</scene> Through van der Waals interactions, Val210 and Arg175 (both orange) also contribute to the specific binding of guanine in this example. These residues force G1 into a conformation where it forms a hydrogen bond to Thr178. The example of PPR10 binding G1 of ATPH exemplifies the general rules by which PPR proteins bind specific nucleotides: firstly, a residue at the 6 position of one repeat (Thr178 in the previous example) forms a hydrogen bond with the base. The identity of this residue determines whether the repeat will bind a purine (adenine and guanine) or pyrimidine (cytosine and uracil). It appears that serine and threonine at position 6 are specific for purines, and asparagine at position 6 is specific for pyrimidines.<ref>doi:10.1371/journal.pgen.1002910</ref> Secondly, a residue at position 1 of the next repeat (Val210 in the previous example) completes the specificity of the interaction. Through van der Waals interactions, this residue determines between A/G and C/U. Other amino acids further contribute to this mechanism, but the previously described rules always apply when PPR proteins bind RNA sequences with modularity.<ref name = "engineering">DOI:10.1111/tpj.12377</ref> | ||
| + | |||
| + | [[Image:PPR10binding.png]] | ||
==Synthetic Applications== | ==Synthetic Applications== | ||
Revision as of 02:20, 9 May 2016
| |||||||||||