This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox WWC7
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<StructureSection load='4OE1' size='350' side='right' caption=''PPR10 structure isolated from ''Zea Mays'' (PDB code [[4OE1]])' > | <StructureSection load='4OE1' size='350' side='right' caption=''PPR10 structure isolated from ''Zea Mays'' (PDB code [[4OE1]])' > | ||
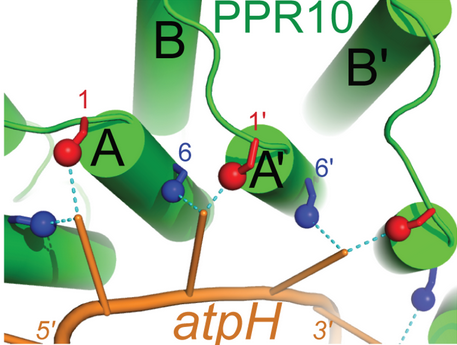
Pentatricopeptide repeat (PPR) proteins are a family of sequence specific RNA-binding proteins which participate in organelle RNA metabolism. Although the mechanisms of RNA binding and the functions of PPR proteins are not fully understood, PPR proteins are thought to assist in RNA editing,<ref>PMID:17015439</ref> translation,<ref name = "translation">DOI:10.1073/pnas.1012076108</ref> and organelle biogenesis.<ref>PMID:15269332</ref> They make up the majority of RNA-binding factors in plant organelles. PPR proteins are characterized by a series of tandem-repeat amino acid consensus sequences which form α-helix <scene name='69/696301/Hairpins/1'>hairpins</scene>. These hairpin structures accumulate to form a α-solenoid tertiary structure. PPR proteins belong to one of two classes: P-class and PLS-class, with the P-class containing 35 amino acid repeats and the PLS-class containing 31-36 amino acid repeats. PPR10 is a well-characterized P-class PPR protein found in the chloroplast of ''Zea mays''.<ref name = "translation"/> | Pentatricopeptide repeat (PPR) proteins are a family of sequence specific RNA-binding proteins which participate in organelle RNA metabolism. Although the mechanisms of RNA binding and the functions of PPR proteins are not fully understood, PPR proteins are thought to assist in RNA editing,<ref>PMID:17015439</ref> translation,<ref name = "translation">DOI:10.1073/pnas.1012076108</ref> and organelle biogenesis.<ref>PMID:15269332</ref> They make up the majority of RNA-binding factors in plant organelles. PPR proteins are characterized by a series of tandem-repeat amino acid consensus sequences which form α-helix <scene name='69/696301/Hairpins/1'>hairpins</scene>. These hairpin structures accumulate to form a α-solenoid tertiary structure. PPR proteins belong to one of two classes: P-class and PLS-class, with the P-class containing 35 amino acid repeats and the PLS-class containing 31-36 amino acid repeats. PPR10 is a well-characterized P-class PPR protein found in the chloroplast of ''Zea mays''.<ref name = "translation"/> | ||
| - | |||
==Function== | ==Function== | ||
In the ''Zea mays'' plastid, PPR10 binds specifically to the ssRNA oligonucleotides ATPH (17 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') and SPAJ (18 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') where it has been shown to shield the transcripts from ribonucleases. In addition to stabilizing these RNA sequences, PPR10 increases the rate at which they are translated.<ref name = "translation"/> | In the ''Zea mays'' plastid, PPR10 binds specifically to the ssRNA oligonucleotides ATPH (17 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') and SPAJ (18 nucleotides: 5'-GUAUUCUUUAAUUAUUUC-3') where it has been shown to shield the transcripts from ribonucleases. In addition to stabilizing these RNA sequences, PPR10 increases the rate at which they are translated.<ref name = "translation"/> | ||
| - | |||
==Mechanism== | ==Mechanism== | ||
| Line 22: | Line 20: | ||
==Synthetic Applications== | ==Synthetic Applications== | ||
There is potential. . . | There is potential. . . | ||
| - | |||
==References== | ==References== | ||
{{Reflist}} | {{Reflist}} | ||
Revision as of 02:45, 9 May 2016
| |||||||||||