Sandbox WWC6
From Proteopedia
| Line 1: | Line 1: | ||
| + | =Hemolysin= | ||
| + | |||
[[Hemolysins]] [https://en.wikipedia.org/wiki/Hemolysin#.CE.B1-hemolysin] are a lipid or protein toxins secreted by pathogens that lyse erythrocyte and some bacterial cell membranes. These toxins belong to a family of microbial exotoxins called cytolysins, which act on a broad number of cells[http://www.uniprot.org/uniprot/P09616]. The primary function of peptide hemolysins is pore formation at the cell membranes creating acytolytic effect, and is achieved by the release of cytosolic ions and small molecules through the hydrophilic, transmembrane portion of the beta-barrel pore[http://www.sciencedirect.com/science/article/pii/S0041010101001532]. | [[Hemolysins]] [https://en.wikipedia.org/wiki/Hemolysin#.CE.B1-hemolysin] are a lipid or protein toxins secreted by pathogens that lyse erythrocyte and some bacterial cell membranes. These toxins belong to a family of microbial exotoxins called cytolysins, which act on a broad number of cells[http://www.uniprot.org/uniprot/P09616]. The primary function of peptide hemolysins is pore formation at the cell membranes creating acytolytic effect, and is achieved by the release of cytosolic ions and small molecules through the hydrophilic, transmembrane portion of the beta-barrel pore[http://www.sciencedirect.com/science/article/pii/S0041010101001532]. | ||
| Line 6: | Line 8: | ||
== Function == | == Function == | ||
| + | Hemolysins are most commonly proteins found in red blood cells that selectively allow for the diffusion of potassium ions across the membrane. <ref >https://en.wikipedia.org/wiki/Hemolysin#cite_note-pmid20692229-3</ref> or lipid biosurfactants that disrupt membrane composition resulting in cell lysis. Hemolysins act through disruption of the cell membrane. <ref>http://www.sciencedirect.com/science/article/pii/S0005273610002610</ref> Pore formation<ref name ="sod"/> is the olgomerization of the pore sunbunits within the membrane. The pore is quickly filled with water, ions, and small molecules that rapidly exit the cell, dissipating ionic gradients and membrane potential. Osmotic pressure causes a rapid swelling of the cell, leading to total rupture of the membrane <ref>http://www.ks.uiuc.edu/Research/hemolysin/<ref>. These proteins are important for some erythrocyte nutrient accession, but cause massive erythrocyte destruction in bacterial infection, specifically responsible forhemolytic anemia, which causes fatigue, pain, arrythmias, and even heart failure in affected individuals.<ref>http://www.nhlbi.nih.gov/health/health-topics/topics/ha/</ref> | ||
| - | Hemolysins are most commonly proteins found in red blood cells that selectively allow for the diffusion of potassium ions across the membrane. <ref >https://en.wikipedia.org/wiki/Hemolysin#cite_note-pmid20692229-3</ref> or lipid biosurfactants that disrupt membrane composition resulting in cell lysis. These proteins are important for some erythrocyte nutrient accession, but cause massive erythrocyte destruction in bacterial infection, specifically responsible forhemolytic anemia, which causes fatigue, pain, arrythmias, and even heart failure in affected individuals.<ref>http://www.nhlbi.nih.gov/health/health-topics/topics/ha/</ref> | ||
| - | |||
| - | |||
| - | Hemolysins act through disruption of the cell membrane. Two main functions destroy phospholipid membranes: pore formation and phosphilipid hydrosysis. <ref>http://www.sciencedirect.com/science/article/pii/S0005273610002610</ref> Pore formation, the most common mechanism of hemolysin cell <ref name ="sod"/> is the olgomerization of the pore sunbunits within the membrane. The pore is quickly filled with water, ions, and small molecules that rapidly exit the cell, dissipating ionic gradients and membrane potential. Osmotic pressure causes a rapid swelling of the cell, leading to total rupture of the membrane <ref>http://www.ks.uiuc.edu/Research/hemolysin/<ref>. | ||
==Structure== | ==Structure== | ||
| Line 22: | Line 21: | ||
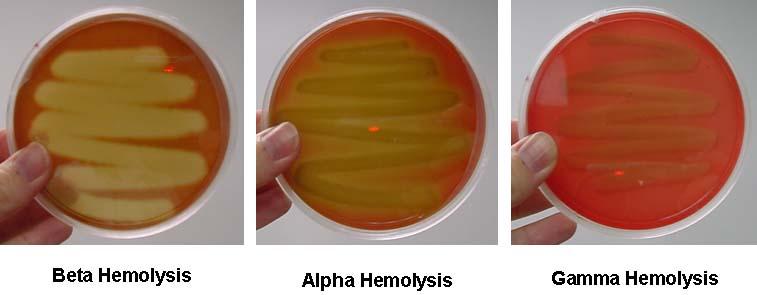
Alpha hemolysins cause a partial lysis of red blood cells. | Alpha hemolysins cause a partial lysis of red blood cells. | ||
The heptameric pore assembles from water-soluble subunits The transmembrane domain of this water-filled pore is primarily comprised of an anti-parallel beta-barrel | The heptameric pore assembles from water-soluble subunits The transmembrane domain of this water-filled pore is primarily comprised of an anti-parallel beta-barrel | ||
| - | |||
| Line 30: | Line 28: | ||
Beta-hemolysins cause a total lysis of red blood cells. | Beta-hemolysins cause a total lysis of red blood cells. | ||
| - | |||
| - | |||
| - | |||
| Line 46: | Line 41: | ||
| - | == Mechanism of action == | + | == Mechanism of action== |
Four of each of the two subunits assemble in an alternating, circular pattern in the γ-HL pore, whereas seven distinct α-HL protomers assemble in a circular arrangement in the α-HL pore. These typically are comprised of three domains: the cap, rim and stem domains, named for the structural resemblance to a mushroom. The cap domain contains β-sandwiches from each protomer, while just below, the rim domain contains four looping β-strands. The stem domain takes on the antiparallel β-barrel, a portion of which becomes the transmembrane structure. | Four of each of the two subunits assemble in an alternating, circular pattern in the γ-HL pore, whereas seven distinct α-HL protomers assemble in a circular arrangement in the α-HL pore. These typically are comprised of three domains: the cap, rim and stem domains, named for the structural resemblance to a mushroom. The cap domain contains β-sandwiches from each protomer, while just below, the rim domain contains four looping β-strands. The stem domain takes on the antiparallel β-barrel, a portion of which becomes the transmembrane structure. | ||
| - | |||
| - | |||
Revision as of 11:07, 13 May 2016
Contents |
Hemolysin
Hemolysins [1] are a lipid or protein toxins secreted by pathogens that lyse erythrocyte and some bacterial cell membranes. These toxins belong to a family of microbial exotoxins called cytolysins, which act on a broad number of cells[2]. The primary function of peptide hemolysins is pore formation at the cell membranes creating acytolytic effect, and is achieved by the release of cytosolic ions and small molecules through the hydrophilic, transmembrane portion of the beta-barrel pore[3].
|
Function
Hemolysins are most commonly proteins found in red blood cells that selectively allow for the diffusion of potassium ions across the membrane. [1] or lipid biosurfactants that disrupt membrane composition resulting in cell lysis. Hemolysins act through disruption of the cell membrane. [2] Pore formation[3] is the olgomerization of the pore sunbunits within the membrane. The pore is quickly filled with water, ions, and small molecules that rapidly exit the cell, dissipating ionic gradients and membrane potential. Osmotic pressure causes a rapid swelling of the cell, leading to total rupture of the membrane [4]
Structure
Hemolysins have three structural variations: alpha, beta, and gamma. These hemolysin types are comprised of di-, hepta- or octomeric subunits.[3] The alpha subunit, depicted right, consists of four repeating structures, named I through IV and shown in different colors .
Alpha-hemolysin
Alpha hemolysins cause a partial lysis of red blood cells. The heptameric pore assembles from water-soluble subunits The transmembrane domain of this water-filled pore is primarily comprised of an anti-parallel beta-barrel
Beta-hemolysin
Beta-hemolysins cause a total lysis of red blood cells.
Gamma-hemolysin
Gamma-hemolysin is both hemolytic and leukotoxic.
Pathogenic Microorganisms
Pore-forming toxins have been shown to closely relate to the pathogenicity of the toxin-producing organism [5]
Oncology
This disease causes seizures, fainting or sudden death from cardiac arrhythmias and is caused my a mutation in the SCN5A gene, or the gene that encodes the NaV1.5 alpha subunit. [6][7] It was found that this deletion includes residues 1505-1507 (KPQ).[6] These residues occur in the cytoplasmic linker between domain III and domain IV. [6]
Hemolytic anemia
Hyperkalemic period paralysis is caused by the mutations T704M, S906T, A1156T, M1360V, A1448C and/or M1592V. [8] These mutations cause periodic or permanent weakness. [8] Physiologically, this is a gain of function mutation. During rest after exercise, or after eating foods rich in K+, the extracellular K+ increases, which mildly depolarizes the membrane.[8] This causes abnormal Na+ channels to open, and they are unable to inactivate. [8] This sustained depolarization of the membrane causes even more abnormal Na+ channels to open and ultimately this leads to loss of excitability and weakness. [8] This symptom usually appears within the first decade of life and can be aggravated by exercise, cold, potassium loading, fasting or pregnancy. [8] Attacks are usually brief and do not need treatment. [8]
Marker for fungi exposure
Many indoor fungi have been shown to produse both alpha and beta-hemolysins. The treatment of blood samples with
Treatment
References
- ↑ https://en.wikipedia.org/wiki/Hemolysin#cite_note-pmid20692229-3
- ↑ http://www.sciencedirect.com/science/article/pii/S0005273610002610
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedsod - ↑ http://www.ks.uiuc.edu/Research/hemolysin/<ref>. These proteins are important for some erythrocyte nutrient accession, but cause massive erythrocyte destruction in bacterial infection, specifically responsible forhemolytic anemia, which causes fatigue, pain, arrythmias, and even heart failure in affected individuals.<ref>http://www.nhlbi.nih.gov/health/health-topics/topics/ha/</li> <li id="cite_note-4">[[#cite_ref-4|↑]] http://www.ncbi.nlm.nih.gov/pubmed/1930675<ref> Both gram positive and gram negative bacteria are producers of hemolysins, as well as some clinically relevant fungi. Toxin secretion facillitates the availability of water, ions, and small molecules like sugar for the secreting pathogen. == Mechanism of action== Four of each of the two subunits assemble in an alternating, circular pattern in the γ-HL pore, whereas seven distinct α-HL protomers assemble in a circular arrangement in the α-HL pore. These typically are comprised of three domains: the cap, rim and stem domains, named for the structural resemblance to a mushroom. The cap domain contains β-sandwiches from each protomer, while just below, the rim domain contains four looping β-strands. The stem domain takes on the antiparallel β-barrel, a portion of which becomes the transmembrane structure. ===Pore formation=== Studies suggest that pore formation is achieved through a nonlytic intermediate oligomer, known as a prepore. The prepore model proposal suggests that the monomeric components assemble on the cell membrane surfacte into a prepore with prestem subunits packed inside. The formed prepore then goes through a conformational change prestem, forming the β-barrel pore. Several issues with the proposed pore formation mechanism have been identified including steric hindrance of the packed prestem structure. [[Image:Ncomms5897-f5.jpg]] This image shows the proposed mechanism of pore formation in the cell membrane. <ref>http://www.nature.com/ncomms/2014/140929/ncomms5897/full/ncomms5897.html<ref> ===Role in infection=== There are nine different α subunits named NaV1.1 through NAV1.9. <ref name = "sod"/> Genes are SCN1 through SCN11. <ref name = "sod"/> These structures differ in their sequence and kinetics. <ref name = "sod"/> As stated above, the α subunit is necessary to the function of the channel and can function independently of the β subunit. You can find the structures and more information below. ==Medical Implications== Diseases caused by mutations in sodium channels can come in many forms. Some mutations affect skeletal, cardiac or smooth muscle, while others affect neural function. Common diseases include long QT syndrome, hyperkalemic periodic paralysis, hypokalemic periodic paralysis, myotonia fluctuans and myotonia permanens among many others. <ref name ="dis">http://neuromuscular.wustl.edu/mother/chan.html#SCN4A</li> <li id="cite_note-QT-5">↑ <sup>[[#cite_ref-QT_5-0|6.0]]</sup> <sup>[[#cite_ref-QT_5-1|6.1]]</sup> <sup>[[#cite_ref-QT_5-2|6.2]]</sup> doi: https://dx.doi.org/10.1016/0092-8674(95)90359-3</li> <li id="cite_note-Long-6">[[#cite_ref-Long_6-0|↑]] http://www.mayoclinic.org/diseases-conditions/long-qt-syndrome/basics/definition/con-20025388</li> <li id="cite_note-Hyper-7">↑ <sup>[[#cite_ref-Hyper_7-0|8.0]]</sup> <sup>[[#cite_ref-Hyper_7-1|8.1]]</sup> <sup>[[#cite_ref-Hyper_7-2|8.2]]</sup> <sup>[[#cite_ref-Hyper_7-3|8.3]]</sup> <sup>[[#cite_ref-Hyper_7-4|8.4]]</sup> <sup>[[#cite_ref-Hyper_7-5|8.5]]</sup> <sup>[[#cite_ref-Hyper_7-6|8.6]]</sup> http://neuromuscular.wustl.edu/mother/activity.html#hrpp</li></ol></ref>