We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:R. Jeremy Johnson/GPR40
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
<includeonly></includeonly>==Human GPR40 (hGPR40), also known as Free Fatty Acid Receptor 1 (FFAR1)== | <includeonly></includeonly>==Human GPR40 (hGPR40), also known as Free Fatty Acid Receptor 1 (FFAR1)== | ||
| - | <StructureSection load='Sele4phu.pdb' size='400' side='right' caption='Human G-Protein Receptor 40 (hGPR40) visualized at 2.3 Å resolution by X-ray crystallography (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=4phu 4PHU]). The natural substrates of this protein are free fatty acids, giving rise to its secondary name, Free Fatty Acid Receptor 1 (FFAR1).' scene='72/727085/Hgpr40_begin/2'> | + | <StructureSection load='Sele4phu.pdb' size='400' side='right' caption='Human G-Protein Receptor 40 (hGPR40) visualized at 2.3 Å resolution by X-ray crystallography (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=4phu 4PHU]). The natural substrates of this protein are free fatty acids, giving rise to its secondary name, Free Fatty Acid Receptor 1 (FFAR1).' scene='72/727085/Hgpr40_begin/2'> |
== Background == | == Background == | ||
| Line 7: | Line 7: | ||
== Function == | == Function == | ||
GPR40 is most prevalent in [https://en.wikipedia.org/wiki/Beta_cell pancreatic β-cells] where free fatty acids (FFAs) have pleiotropic effects <ref name="FFA">PMID:19460454</ref>. While acute intake of FFAs stimulates insulin release, chronic exposure to high levels of FFAs results in the impairment of β-cell function and insulin secretory response <ref name= "FFA"/>. GPR40 mediates the effect of both acute and chronic levels of FFAs. FFAs amplify glucose-stimulated insulin secretion from pancreatic β-cells by activating GPR40. When GPR40 is inhibited, insulin secretion no longer increases in response to fatty acid stimulation <ref name= "FFA"/>. This decreased activity of GPR40 leads to a decreased risk of [https://en.wikipedia.org/wiki/Hyperinsulinemia hyperinsulinemia], [https://en.wikipedia.org/wiki/Fatty_liver fatty liver disease], [https://en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia], [https://en.wikipedia.org/wiki/Hyperglycemia hyperglycemia], and [https://en.wikipedia.org/wiki/Impaired_glucose_tolerance glucose tolerance] in obese patients <ref name= "FFA"/>. On the contrary, overexpression of GPR40 leads to impaired β-cell function, hyperinsulinemia, and diabetes <ref name= "FFA"/>. These results suggest that GPR40 plays an important role in the mechanism that links obesity and type 2 diabetes and thus is a popular drug target being studied. | GPR40 is most prevalent in [https://en.wikipedia.org/wiki/Beta_cell pancreatic β-cells] where free fatty acids (FFAs) have pleiotropic effects <ref name="FFA">PMID:19460454</ref>. While acute intake of FFAs stimulates insulin release, chronic exposure to high levels of FFAs results in the impairment of β-cell function and insulin secretory response <ref name= "FFA"/>. GPR40 mediates the effect of both acute and chronic levels of FFAs. FFAs amplify glucose-stimulated insulin secretion from pancreatic β-cells by activating GPR40. When GPR40 is inhibited, insulin secretion no longer increases in response to fatty acid stimulation <ref name= "FFA"/>. This decreased activity of GPR40 leads to a decreased risk of [https://en.wikipedia.org/wiki/Hyperinsulinemia hyperinsulinemia], [https://en.wikipedia.org/wiki/Fatty_liver fatty liver disease], [https://en.wikipedia.org/wiki/Hypertriglyceridemia hypertriglyceridemia], [https://en.wikipedia.org/wiki/Hyperglycemia hyperglycemia], and [https://en.wikipedia.org/wiki/Impaired_glucose_tolerance glucose tolerance] in obese patients <ref name= "FFA"/>. On the contrary, overexpression of GPR40 leads to impaired β-cell function, hyperinsulinemia, and diabetes <ref name= "FFA"/>. These results suggest that GPR40 plays an important role in the mechanism that links obesity and type 2 diabetes and thus is a popular drug target being studied. | ||
| + | |||
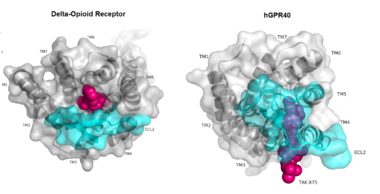
| + | [[Image:Gpcr comparison.png|380 px|thumb|center|'''Figure 1:'''Comparison of Delta-opioid receptor to human free-fatty acid receptor (hGPR40) both of which are G-protein coupled receptors. The binding pocket of the delta-opioid receptor is solvent exposed allowing ligands to enter directly from the extracellular space while the binding pocket of hGPR40 is covered by the extracellular loop 2 (ECL2) preventing entry from the extracellular space (ECL2 represented in cyan). The Delta-opioid displays the canonical binding site typical of most GPCRs while ligands of hGPR40 bind to a noncanonical pocket represented in pink.]] | ||
== Structure == | == Structure == | ||
| Line 12: | Line 14: | ||
While there is relatively low sequence identity between hGPR40 and peptide-binding and [https://en.wikipedia.org/wiki/Opioid_receptor opioid GPCRs], they do share structural similarities such as a conserved <scene name='72/727085/Hairpin_loop/4'>hairpin loop</scene> motif on <scene name='72/727085/Ecl2/4'>extracellular loop 2 </scene>(ECL2)<ref name="crystal"/>. In addition, there is a conserved <scene name='72/727085/Disulfide/3'>disulphide bond</scene> that is formed between transmembrane helix 3 (Cys 79) and the C-terminus of ECL2 (Cys170)<ref name="crystal"/>. Compared to peptide-binding and opioid GPCRs which have distinctive [https://en.wikipedia.org/wiki/Beta_sheet β-sheets] spanning from transmembrane helix 4 to 5, hGPR40 possesses a shorter B-sheet-like region which has [http://proteopedia.org/wiki/index.php/Image:Beta-like_factors_of_hGPR40_ECL2.png low B-factors]<ref name="crystal"/>. This reflects the low mobility of the region that limits the overall flexibility of the adjacent portion of ECL2 between Leu171 and Asp175<ref name="crystal"/>. A unique feature of hGPR40 is the presence of an additional 13 residues (Pro147 to Gly159) on ECL2 which is absent on all the other peptide/opioid receptors<ref name="crystal"/>. These extra residues form a separate <scene name='72/727085/Auxiliary_loop/3'>auxiliary loop</scene> between the B-sheet-like region and transmembrane 4. Together, the auxiliary loop and ECL2 of hGPR40 function as a <scene name='72/727085/Ecl2_cap/3'>roof </scene> over the canonical binding site covering it from the central extracellular region<ref name="crystal"/>. | While there is relatively low sequence identity between hGPR40 and peptide-binding and [https://en.wikipedia.org/wiki/Opioid_receptor opioid GPCRs], they do share structural similarities such as a conserved <scene name='72/727085/Hairpin_loop/4'>hairpin loop</scene> motif on <scene name='72/727085/Ecl2/4'>extracellular loop 2 </scene>(ECL2)<ref name="crystal"/>. In addition, there is a conserved <scene name='72/727085/Disulfide/3'>disulphide bond</scene> that is formed between transmembrane helix 3 (Cys 79) and the C-terminus of ECL2 (Cys170)<ref name="crystal"/>. Compared to peptide-binding and opioid GPCRs which have distinctive [https://en.wikipedia.org/wiki/Beta_sheet β-sheets] spanning from transmembrane helix 4 to 5, hGPR40 possesses a shorter B-sheet-like region which has [http://proteopedia.org/wiki/index.php/Image:Beta-like_factors_of_hGPR40_ECL2.png low B-factors]<ref name="crystal"/>. This reflects the low mobility of the region that limits the overall flexibility of the adjacent portion of ECL2 between Leu171 and Asp175<ref name="crystal"/>. A unique feature of hGPR40 is the presence of an additional 13 residues (Pro147 to Gly159) on ECL2 which is absent on all the other peptide/opioid receptors<ref name="crystal"/>. These extra residues form a separate <scene name='72/727085/Auxiliary_loop/3'>auxiliary loop</scene> between the B-sheet-like region and transmembrane 4. Together, the auxiliary loop and ECL2 of hGPR40 function as a <scene name='72/727085/Ecl2_cap/3'>roof </scene> over the canonical binding site covering it from the central extracellular region<ref name="crystal"/>. | ||
| - | |||
| - | [[Image:Gpcr comparison.png|380 px|thumb|center|'''Figure 1:'''Comparison of Delta-opioid receptor to human free-fatty acid receptor (hGPR40) both of which are G-protein coupled receptors. The binding pocket of the delta-opioid receptor is solvent exposed allowing ligands to enter directly from the extracellular space while the binding pocket of hGPR40 is covered by the extracellular loop 2 (ECL2) preventing entry from the extracellular space (ECL2 represented in cyan). The Delta-opioid displays the canonical binding site typical of most GPCRs while ligands of hGPR40 bind to a noncanonical pocket represented in pink.]] | ||
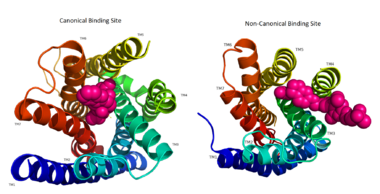
The canonical binding pocket for many other GPCRs is solvent exposed and centrally located between the transmembrane helices allowing ligands to directly bind from the extracellular space<ref name="crystal"/>. However, because the ECL2 acts as a roof to this canonical binding site, it inhibits ligands from entering directly from the extracellular region. Instead, the highly lipophilic nature of hGPRC40’s ligands allow it to enter a <scene name='72/727085/Hgpr40_entry/2'>noncanonical binding pocket </scene> between TM3 and TM4 by moving through the lipid bilayer<ref name="crystal"/>. | The canonical binding pocket for many other GPCRs is solvent exposed and centrally located between the transmembrane helices allowing ligands to directly bind from the extracellular space<ref name="crystal"/>. However, because the ECL2 acts as a roof to this canonical binding site, it inhibits ligands from entering directly from the extracellular region. Instead, the highly lipophilic nature of hGPRC40’s ligands allow it to enter a <scene name='72/727085/Hgpr40_entry/2'>noncanonical binding pocket </scene> between TM3 and TM4 by moving through the lipid bilayer<ref name="crystal"/>. | ||
| Line 35: | Line 35: | ||
hGPR40 functions as a free fatty acid receptor that participates in [http://www.abcam.com/pathways/overview-of-insulin-signaling-pathways insulin signaling] to regulate blood glucose concentrations. The actual mechanisms by which insulin signaling occurs are unknown, but multiple theoretical mechanisms have been posited for how hGPR40 participates in the regulation of glucose uptake.<ref name="Srivastava"/> | hGPR40 functions as a free fatty acid receptor that participates in [http://www.abcam.com/pathways/overview-of-insulin-signaling-pathways insulin signaling] to regulate blood glucose concentrations. The actual mechanisms by which insulin signaling occurs are unknown, but multiple theoretical mechanisms have been posited for how hGPR40 participates in the regulation of glucose uptake.<ref name="Srivastava"/> | ||
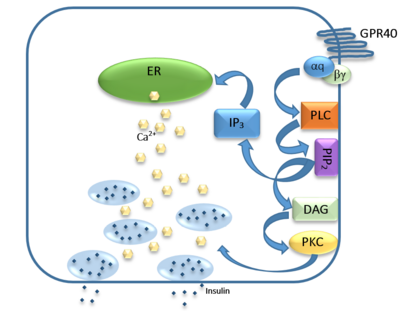
| - | [[Image:Gpr40 insulin pathway.png|400 px|center|thumb|'''Figure | + | [[Image:Gpr40 insulin pathway.png|400 px|center|thumb|'''Figure 3:''' Signaling pathway mediated by GPR40 in pancreatic β-cells. The 𝝰q subunit represents the Gq unit that is coupled to hGPR40. This subunit breaks off to activate phospholipase C (PLC), resulting in the hydrolysis of phosphatidylinositol-4,5-biphosphate (PIP<sub>2</sub>). PIP<sub>2</sub> goes on to create diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). The DAG activates protein kinase c (PKC), triggering secretion of insulin. Activated IP3 diffuses to the endoplasmic reticulum (ER), releasing the stored Ca<sup>2+</sup>, which aids in insulin secretion. ]] |
The natural substrate of hGPR40 is free fatty acids (FFAs) which bind to the G-protein and enhance glucose-stimulated insulin secretion <ref name="Morg">PMID:19660440</ref>. FFAs bind to GPR40 which then couples with the G-protein Gq leading to increased [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] (PLC) activity<ref name="Morg"/>. PLC catalyzes the hydrolysis of the phospholipid [https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate phosphatidylinositol-4,5-biphosphate ] (PIP<sub>2</sub>) resulting in the formation of [https://en.wikipedia.org/wiki/Diglyceride diacylglycerol] (DAG) and [https://en.wikipedia.org/wiki/Inositol_trisphosphate inositol 1,4,5-triphosphate] (IP3)<ref name="Morg"/>. DAG can then activate [https://en.wikipedia.org/wiki/Protein_kinase_C protein kinase C] (PKC) to enhance insulin secretion<ref name="Morg"/>. IP3 on the other hand is soluble and diffuses to the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum] where it binds a ligand-gated Ca<sup>2+</sup> channel<ref name="Morg"/>. This binding triggers the opening of the channel causing stored Ca<sup>2+</sup> to be released into the cytoplasm<ref name="Morg"/>. This large increase in intracellular free Ca<sup>2+</sup> produces a proportional increase in glucose-dependent insulin secretion, suggesting that insulin release can be contributed in part to the changes in Ca<sup>2+</sup> concentration resulting from activated GPR40 <ref name="Morg"/>. | The natural substrate of hGPR40 is free fatty acids (FFAs) which bind to the G-protein and enhance glucose-stimulated insulin secretion <ref name="Morg">PMID:19660440</ref>. FFAs bind to GPR40 which then couples with the G-protein Gq leading to increased [https://en.wikipedia.org/wiki/Phospholipase_C phospholipase C] (PLC) activity<ref name="Morg"/>. PLC catalyzes the hydrolysis of the phospholipid [https://en.wikipedia.org/wiki/Phosphatidylinositol_4,5-bisphosphate phosphatidylinositol-4,5-biphosphate ] (PIP<sub>2</sub>) resulting in the formation of [https://en.wikipedia.org/wiki/Diglyceride diacylglycerol] (DAG) and [https://en.wikipedia.org/wiki/Inositol_trisphosphate inositol 1,4,5-triphosphate] (IP3)<ref name="Morg"/>. DAG can then activate [https://en.wikipedia.org/wiki/Protein_kinase_C protein kinase C] (PKC) to enhance insulin secretion<ref name="Morg"/>. IP3 on the other hand is soluble and diffuses to the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum] where it binds a ligand-gated Ca<sup>2+</sup> channel<ref name="Morg"/>. This binding triggers the opening of the channel causing stored Ca<sup>2+</sup> to be released into the cytoplasm<ref name="Morg"/>. This large increase in intracellular free Ca<sup>2+</sup> produces a proportional increase in glucose-dependent insulin secretion, suggesting that insulin release can be contributed in part to the changes in Ca<sup>2+</sup> concentration resulting from activated GPR40 <ref name="Morg"/>. | ||
| Line 43: | Line 43: | ||
=== [http://proteopedia.org/wiki/index.php/Image:Tak_875.png TAK-875] === | === [http://proteopedia.org/wiki/index.php/Image:Tak_875.png TAK-875] === | ||
| - | + | <scene name='72/727085/Hgpr40_begin/3'>Tak-875</scene> is a [https://en.wikipedia.org/wiki/Partial_agonist partial agonist] of GPR40 and tested for the treatment of type 2 diabetes. The binding of TAK-875 to hGPR40 occurs by the ligand entering the binding site through the [https://en.wikipedia.org/wiki/Cell_membrane membrane bilayer]. This membrane insertion is performed via a method similar to ligand binding to [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate_receptor sphingosine 1-phosphate receptor 1], retinal loading of [http://proteopedia.org/wiki/index.php/4j4q GPCR opsin], and the entry of anandamide in [https://en.wikipedia.org/wiki/Cannabinoid_receptor cannabinoid receptors], in which the <scene name='72/727085/Ecl2/4'>extracellular loops</scene> block the binding from the extracellular matrix <ref>PMID:22344443</ref>. The binding mechanism through the bilayer may be selectively favoring the free fatty acid because of the [https://en.wikipedia.org/wiki/Chemical_polarity#Nonpolar_molecules non-polar] regions of the ligand (Figure 5). | |
| - | <scene name='72/727085/Hgpr40_begin/3'>Tak-875</scene> is a [https://en.wikipedia.org/wiki/Partial_agonist partial agonist] of GPR40 and tested for the treatment of type 2 diabetes. The binding of TAK-875 to hGPR40 occurs by the ligand entering the binding site through the [https://en.wikipedia.org/wiki/Cell_membrane membrane bilayer]. This membrane insertion is performed via a method similar to ligand binding to [https://en.wikipedia.org/wiki/Sphingosine-1-phosphate_receptor sphingosine 1-phosphate receptor 1], retinal loading of [http://proteopedia.org/wiki/index.php/4j4q GPCR opsin], and the entry of anandamide in [https://en.wikipedia.org/wiki/Cannabinoid_receptor cannabinoid receptors], in which the <scene name='72/727085/Ecl2/4'>extracellular loops</scene> block the binding from the extracellular matrix <ref>PMID:22344443</ref>. The binding mechanism through the bilayer may be selectively favoring the free fatty acid because of the [https://en.wikipedia.org/wiki/Chemical_polarity#Nonpolar_molecules non-polar] regions of the ligand (Figure | + | [[Image:Hydrophobic crop.png|380 px|center|thumb|'''Figure 4:''' TAK-875 buries its polar head group within a very hydrophobic region and coordinate within the polar charge network of hGPR40.]] |
| - | [[Image:Hydrophobic crop.png|380 px|center|thumb|'''Figure | + | |
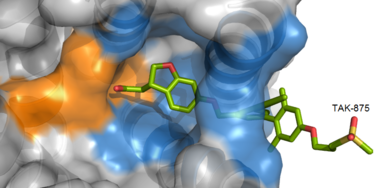
TAK-875 binds to the <scene name='72/727085/Hgpr40_entry/2'>noncanonical binding site </scene> created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. The carboxylate of TAK-875 is buried within a very hydrophobic region and in a complex complex <scene name='72/727085/Hgpr40_binding_relay/6'>charge network</scene> involving Glu172, Ser187, Asn241, and Asn 244 from hGPR40 forming ionic and polar interactions by coordinating TAK-875 with Arg183, Arg258, Tyr91, and Tyr240. | TAK-875 binds to the <scene name='72/727085/Hgpr40_entry/2'>noncanonical binding site </scene> created between transmembrane (TM) domains 3-5 and the extracellular loop 2 (ECL2) of hGPR40. The ECL2 and auxiliary loop form a roof causing TAK-875 to enter through TM3 and TM4, first passing through the lipid bilayer. The carboxylate of TAK-875 is buried within a very hydrophobic region and in a complex complex <scene name='72/727085/Hgpr40_binding_relay/6'>charge network</scene> involving Glu172, Ser187, Asn241, and Asn 244 from hGPR40 forming ionic and polar interactions by coordinating TAK-875 with Arg183, Arg258, Tyr91, and Tyr240. | ||
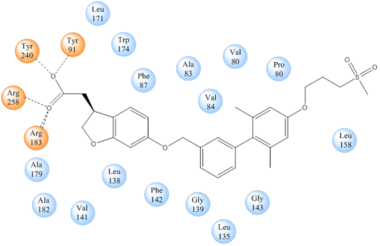
| - | [[Image:Simplified hydrophibic.png|380 px|center|thumb|'''Figure | + | [[Image:Simplified hydrophibic.png|380 px|center|thumb|'''Figure 5:''' Stabilizing binding interactions between TAK-875 and hGPR40. Amino acids denoted in orange coordinate with the polar head of TAK-875 by polar/ionic interactions while blue amino acids stabilize the binding through hydrophobic interactions.]] |
=== Other Potential Inhibitors === | === Other Potential Inhibitors === | ||
| - | + | TAK-875 had the most promising outlooks out of any current known agonists of hGPR40, but it was discontinued. Some other agonists tested in clinical trials include AMG-837 and AM-1638. When coadministered, AMG-837 and AM-1638 enhanced glucose tolerance, but they were found to be toxic in the human trials. Some other agonsits are currently being examined as well. One compound, LY 2881835 (Eli Lilly & Company, Indianapolis, IN), has undergone clinical trials, but the results are unknown. In addition to the above-mentioned compound, other orally bioavailable GPR40-specific agonists are currently in preclinical or clinical development. As of 2015, TUG-770 and CNX-011-67 (Connexios Life Sciences, Karnataka, India) were in preclinical trials and JTT-851 (Japan Tobacco, Toyko, Japan), and P11187 (Piramal, Mumbai, India) were in clinical trails.<ref name="Mancini">PMID: 25604916</ref> | |
| - | + | ||
| - | + | ||
| - | + | ||
| + | == References == | ||
| + | <references/> | ||
</StructureSection> | </StructureSection> | ||
| - | == References == | ||
| - | <references/> | ||
Revision as of 19:52, 9 June 2016
Human GPR40 (hGPR40), also known as Free Fatty Acid Receptor 1 (FFAR1)
| |||||||||||