This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
P53
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
{{Clear}} | {{Clear}} | ||
| - | '''p53 Tumor Suppressor Protein (PDB code [[1tup]])''' | + | __TOC__ |
| + | =='''p53 Tumor Suppressor Protein (PDB code [[1tup]])'''== | ||
---- | ---- | ||
| Line 31: | Line 32: | ||
See also [[P53 (hebrew)]]. | See also [[P53 (hebrew)]]. | ||
| - | '''p53 Pathway and Mutation''' | + | =='''p53 Pathway and Mutation'''== |
In a normal cell, p53 is inactivated by its negative regulatory mdm2 (hdm2 in humans) and it is found at low levels. When DNA damage is sensed, p53's level rises. p53 binds to many regulatory sites in the genome and begins production of proteins that stop cell division until the damage is repaired. If the damage is irreparable, p53 initiates the process called programmed cell death, apoptosis, permanently removing the damage. | In a normal cell, p53 is inactivated by its negative regulatory mdm2 (hdm2 in humans) and it is found at low levels. When DNA damage is sensed, p53's level rises. p53 binds to many regulatory sites in the genome and begins production of proteins that stop cell division until the damage is repaired. If the damage is irreparable, p53 initiates the process called programmed cell death, apoptosis, permanently removing the damage. | ||
| - | |||
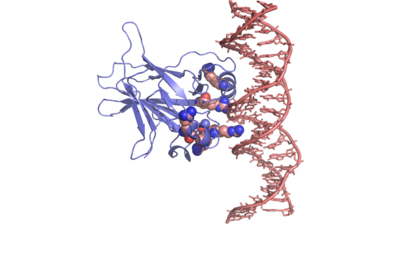
In most cases of human cancer, p53 mutations have been observed. Most of the p53 mutations that may result in cancer are found in and around the DNA-binding surface of the protein. The most common mutation changes R248, an amino acid that interacts with DNA. When mutated to another amino acid, this interaction is lost. Other residues associated with cancer-causing mutations are arginine 175, 249, 273, 282 and glycine 245. The figure at the right shows interaction of the DNA-binding domain with DNA. Key residues associated with mutations are represented by spheres. | In most cases of human cancer, p53 mutations have been observed. Most of the p53 mutations that may result in cancer are found in and around the DNA-binding surface of the protein. The most common mutation changes R248, an amino acid that interacts with DNA. When mutated to another amino acid, this interaction is lost. Other residues associated with cancer-causing mutations are arginine 175, 249, 273, 282 and glycine 245. The figure at the right shows interaction of the DNA-binding domain with DNA. Key residues associated with mutations are represented by spheres. | ||
| Line 42: | Line 42: | ||
{{Clear}} | {{Clear}} | ||
| - | '''Surface charge of the DNA binding domain''' | + | =='''Surface charge of the DNA binding domain'''== |
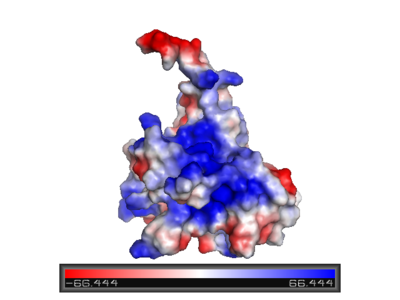
The figure at the left shows the surface charge of the p53 DNA-binding domain. It is rich in arginine amino acids that interact with DNA, and this causes its surface to be positively charged. This domain recognizes specific regulatory sites on the DNA. The flexible structure of p53 allows it to bind to many different variants of binding sites, allowing it to regulate transcription at many places in the genome. | The figure at the left shows the surface charge of the p53 DNA-binding domain. It is rich in arginine amino acids that interact with DNA, and this causes its surface to be positively charged. This domain recognizes specific regulatory sites on the DNA. The flexible structure of p53 allows it to bind to many different variants of binding sites, allowing it to regulate transcription at many places in the genome. | ||
Revision as of 09:49, 13 June 2016
| |||||||||||
3D structures of p53 (Updated on 13-June-2016)
Additional Resources
References
- ↑ Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003 Apr;10(4):431-42. PMID:12719720 doi:http://dx.doi.org/10.1038/sj.cdd.4401183
- ↑ Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010 Feb;2(2):a001107. doi:, 10.1101/cshperspect.a001107. PMID:20182618 doi:http://dx.doi.org/10.1101/cshperspect.a001107
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Michal Harel, Eran Hodis, Mary Ball, Alexander Berchansky, David Canner