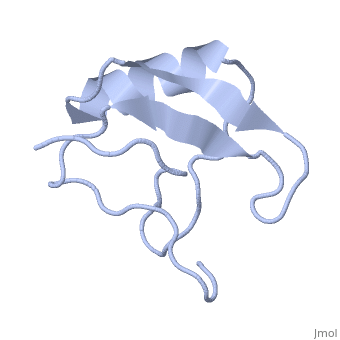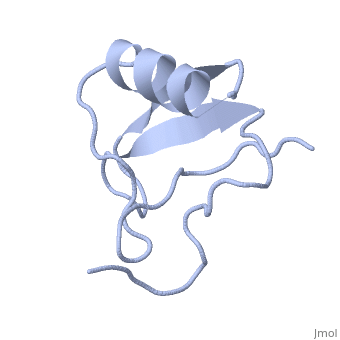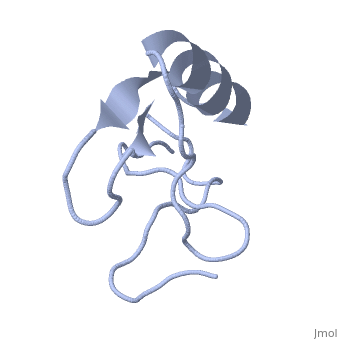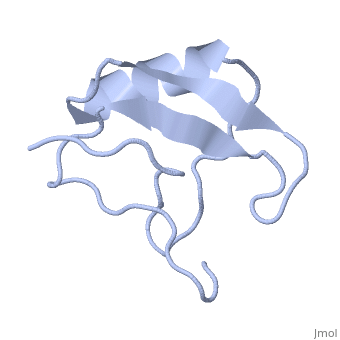
Potassium channel toxin
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==Overview== | ==Overview== | ||
| - | Scorpion toxins are 61- to 76-residue long proteins that modulate the gating properties of voltage-gated sodium channels. According to their mode of action and binding properties, | + | Scorpion toxins are 61- to 76-residue long proteins that modulate the gating properties of voltage-gated sodium channels. According to their mode of action and binding properties, scorpion toxins are divided into two major classes, α- and β- toxins. |
LqhαIT is a 64-residue scorpion α-toxin from [http://en.wikipedia.org/wiki/Deathstalker_scorpion Leiurus quinquestriatus hebraeus] (yellow scorpion) venom. Scorpion α-toxins prolong the action potential by inhibiting channel inactivation. The binding site of scorpion α-neurotoxins on voltage-gated sodium channels is named neurotoxin receptor site-3. | LqhαIT is a 64-residue scorpion α-toxin from [http://en.wikipedia.org/wiki/Deathstalker_scorpion Leiurus quinquestriatus hebraeus] (yellow scorpion) venom. Scorpion α-toxins prolong the action potential by inhibiting channel inactivation. The binding site of scorpion α-neurotoxins on voltage-gated sodium channels is named neurotoxin receptor site-3. | ||
Revision as of 09:11, 10 July 2016
| |||||||||||
3D structures of potassium channel toxins
Updated on 10-July-2016