Protein kinase Spk1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
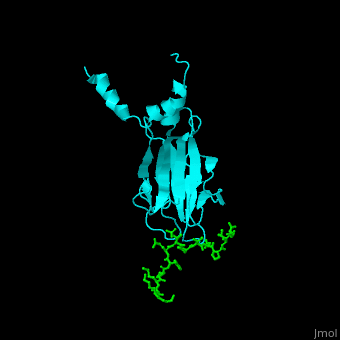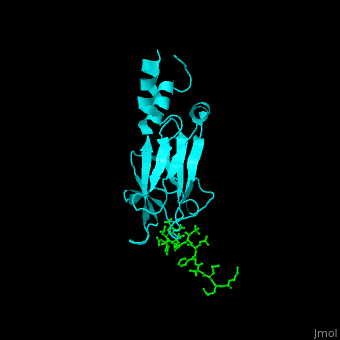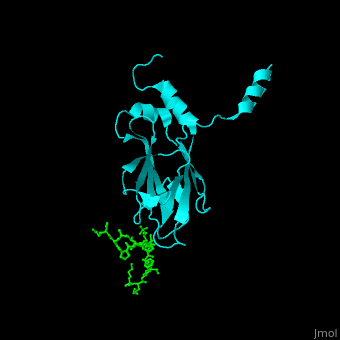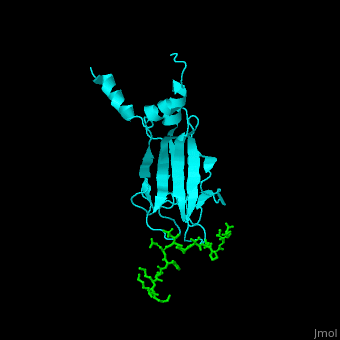
| - | <StructureSection load='1k3n' size='400' side='right' caption='Structure of yeast Rad53 FHA1 domain ( | + | <StructureSection load='1k3n' size='400' side='right' caption='Structure of yeast Rad53 FHA1 domain (cyan) complex with phosphothreonine peptide (green) (PDB code [[1k3n]]).' scene='59/590826/Cv/1'> |
| Line 8: | Line 8: | ||
== Structural highlights == | == Structural highlights == | ||
| - | Rad53 contains phosphothreonine (PTO) recognition domains: '''FHA1''' at the N-terminal (residues 1-164) which selects for Asp at position +3 relative to PTO and '''FHA2''' at the C-terminal (residues 573-730) which selects for Ile at position +3 relative to PTO. Two '''SCD''' - SQ/TQ-rich cluster domains – are flanking the '''kinase''' domain. SCD domain is associated with DNA-damage-response proteins. The yeast FHA1 domain interacts with peptide containing PTOXXD sequence<ref>PMID:11846567</ref>. | + | Rad53 contains phosphothreonine (PTO) recognition domains: '''FHA1''' at the N-terminal (residues 1-164) which selects for Asp at position +3 relative to PTO and '''FHA2''' at the C-terminal (residues 573-730) which selects for Ile at position +3 relative to PTO. Two '''SCD''' - SQ/TQ-rich cluster domains – are flanking the '''kinase''' domain. SCD domain is associated with DNA-damage-response proteins. The <scene name='59/590826/Cv/3'>yeast FHA1 domain interacts with peptide containing PTOXXD sequence</scene><ref>PMID:11846567</ref>. |
</StructureSection> | </StructureSection> | ||
Revision as of 10:04, 25 July 2016
| |||||||||||
3D structures of protein kinase Spk1
Updated on 25-July-2016
References
- ↑ Stern DF, Zheng P, Beidler DR, Zerillo C. Spk1, a new kinase from Saccharomyces cerevisiae, phosphorylates proteins on serine, threonine, and tyrosine. Mol Cell Biol. 1991 Feb;11(2):987-1001. PMID:1899289
- ↑ Yuan C, Yongkiettrakul S, Byeon IJ, Zhou S, Tsai MD. Solution structures of two FHA1-phosphothreonine peptide complexes provide insight into the structural basis of the ligand specificity of FHA1 from yeast Rad53. J Mol Biol. 2001 Nov 30;314(3):563-75. PMID:11846567 doi:10.1006/jmbi.2001.5140