Polyneuridine Aldehyde Esterase
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
Up to now the phytochemical analysis of ''Rauvolfia'' in vitro systems resulted in the isolation of more than 30 monoterpenoid indole alkaloids. Many of them belong to the well known ajmalan-sarpagan type alkaloids. One of the most intriguing alkaloid was '''ajmaline''', a compound comprising a complex hexacyclic ring system bearing nine chiral carbon centers. | Up to now the phytochemical analysis of ''Rauvolfia'' in vitro systems resulted in the isolation of more than 30 monoterpenoid indole alkaloids. Many of them belong to the well known ajmalan-sarpagan type alkaloids. One of the most intriguing alkaloid was '''ajmaline''', a compound comprising a complex hexacyclic ring system bearing nine chiral carbon centers. | ||
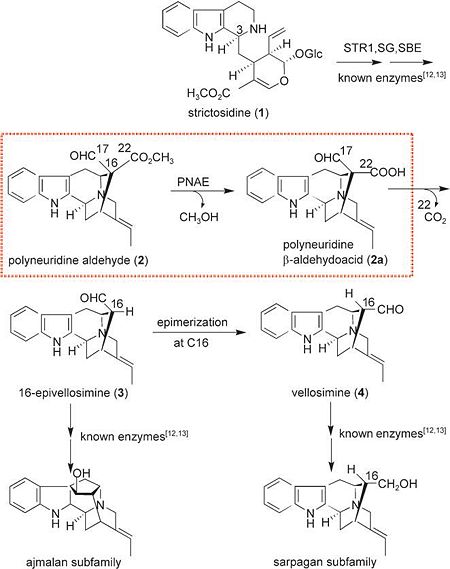
| - | '''Polyneuridine Aldehyde Esterase''''''(PNAE)''' is an important enzyme during Ajmaline Biosynthesis, transforming C-10 skeleton polyneuridine aldehyde into C-9 skeleton epi-vellosimine<ref>PMID:12071952</ref>.[[Image:Pathway.JPG]] | + | '''Polyneuridine Aldehyde Esterase''''''(PNAE)''' is an important enzyme during Ajmaline Biosynthesis, transforming C-10 skeleton polyneuridine aldehyde into C-9 skeleton epi-vellosimine<ref>PMID:12071952</ref>. |
| + | |||
| + | [[Image:Pathway.JPG|left|450px|thumb]] | ||
For the biochemical characterization of '''PNAE''', the enzyme was isolated from plant cell cultures of the Indian medicinal plant ''Rauvolfia serpentina'' and partially sequenced; the '''PNAE''' cDNA was then cloned and overexpressed in ''Escherichia coli''. | For the biochemical characterization of '''PNAE''', the enzyme was isolated from plant cell cultures of the Indian medicinal plant ''Rauvolfia serpentina'' and partially sequenced; the '''PNAE''' cDNA was then cloned and overexpressed in ''Escherichia coli''. | ||
| Line 14: | Line 16: | ||
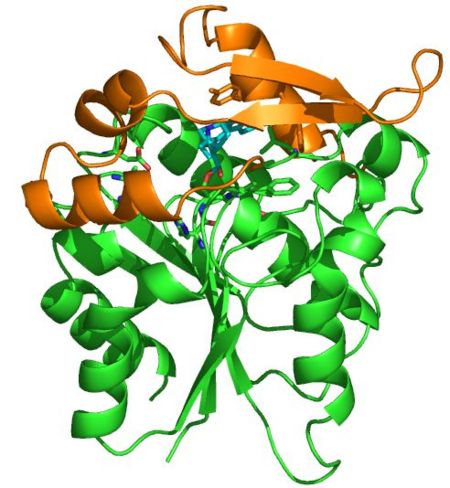
Sequence analysis led to the preliminary classification of '''PNAE''' as a new candidate of the large α/β hydrolase superfamily. This classification is based in particular on the catalytic triad Ser87, Asp216, and His244, which was previously verified by single mutations and homology modeling. | Sequence analysis led to the preliminary classification of '''PNAE''' as a new candidate of the large α/β hydrolase superfamily. This classification is based in particular on the catalytic triad Ser87, Asp216, and His244, which was previously verified by single mutations and homology modeling. | ||
| - | [[Image:F1-1.jpg|left| | + | [[Image:F1-1.jpg|left|450px|thumb]] |
{{clear}} | {{clear}} | ||
The structural data now available for '''PNAE''' support the overall mechanism, which undoubtedly represents the key reaction for the biosynthesis of C9-monoterpenoid ''Rauvolfia'' alkaloids. The data will also allow a rational, structure-based redesign of '''PNAE''', similar what we have recently demonstrated for the Pictet-Spenglerase, strictosidine synthase, which is now applied for chemoenzymatic synthesis of novel alkaloid libraries. | The structural data now available for '''PNAE''' support the overall mechanism, which undoubtedly represents the key reaction for the biosynthesis of C9-monoterpenoid ''Rauvolfia'' alkaloids. The data will also allow a rational, structure-based redesign of '''PNAE''', similar what we have recently demonstrated for the Pictet-Spenglerase, strictosidine synthase, which is now applied for chemoenzymatic synthesis of novel alkaloid libraries. | ||
Revision as of 10:08, 8 August 2016
| |||||||||||
3D structures of polyneuridine aldehyde esterase
2wfm – SePAE (mutant) – Serpentwood
2wfl – SePAE
3gzj – SePAE + 16-epi-vellosimine
References
- ↑ Mattern-Dogru E, Ma X, Hartmann J, Decker H, Stockigt J. Potential active-site residues in polyneuridine aldehyde esterase, a central enzyme of indole alkaloid biosynthesis, by modelling and site-directed mutagenesis. Eur J Biochem. 2002 Jun;269(12):2889-96. PMID:12071952
- ↑ Yang L, Hill M, Wang M, Panjikar S, Stockigt J. Structural basis and enzymatic mechanism of the biosynthesis of C9- from C10-monoterpenoid indole alkaloids. Angew Chem Int Ed Engl. 2009;48(28):5211-3. PMID:19496101 doi:10.1002/anie.200900150
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Michal Harel, Liuqing Yang, Joel L. Sussman