We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Alcohol dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
| - | Within alcohol dehydrogenase, <scene name='Birrer_Sandbox_2/The_active_site/1'>the active</scene> site of alcohol dehydrogenase has three important residues, Phe 93, Leu 57, and Leu 116. These three residues work together to bind to the alcohol substrate.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | + | Within alcohol dehydrogenase, <scene name='Birrer_Sandbox_2/The_active_site/1'>the active</scene> site of alcohol dehydrogenase has three important residues, Phe 93, Leu 57, and Leu 116. These three residues work together to bind to the alcohol substrate.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> |
| - | Zn plays an important role in the catalysis. It funtions by electrostatically stabilizing the oxygen in alcohol during the reaction, which causes the alcohol to be more acidic. At the <scene name='Birrer_Sandbox_2/Zinc_binding_site/1'>Zinc Binding Site</scene>, Zinc coordinates with Cys 146, Cys 174, and His 67.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | + | Zn plays an important role in the catalysis. It funtions by electrostatically stabilizing the oxygen in alcohol during the reaction, which causes the alcohol to be more acidic. At the <scene name='Birrer_Sandbox_2/Zinc_binding_site/1'>Zinc Binding Site</scene>, Zinc coordinates with Cys 146, Cys 174, and His 67.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 <http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> |
NAD functions as a cosubstrate in the dehydration. NAD binds to numerous residues in a series of beta-alpha-beta folds. <scene name='Birrer_Sandbox_2/Nad_binding_site/1'>NAD Binding Region</scene> shows the domain where NAD binds, and many of the residues with which it interacts are selected. | NAD functions as a cosubstrate in the dehydration. NAD binds to numerous residues in a series of beta-alpha-beta folds. <scene name='Birrer_Sandbox_2/Nad_binding_site/1'>NAD Binding Region</scene> shows the domain where NAD binds, and many of the residues with which it interacts are selected. | ||
| - | <ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm | + | <ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm</ref> |
| - | Alcohol dehydrogenase exists as a dimer with a zinc molecule complexed in each of the subunits. It has a SCOP catagory of an alpha and beta protein. At the N-terminal, there is a domain that is all beta; however, the C-Terminal domain is alpha and beta, so the catagory is alpha and beta. The C-Terminal core has 3 layers of alpha/beta/alpha and parallel beta sheets of 6 strands.<ref>''Protein: Alcohol dehydrogenase from Human (Homo sapiens), different isozymes''. SCOP. 2009. 1 March 2010 < http://scop.berkeley.edu/data/scop.b.d.c.b.b.c.html></ref> | + | Alcohol dehydrogenase exists as a dimer with a zinc molecule complexed in each of the subunits. It has a SCOP catagory of an alpha and beta protein. At the N-terminal, there is a domain that is all beta; however, the C-Terminal domain is alpha and beta, so the catagory is alpha and beta. The C-Terminal core has 3 layers of alpha/beta/alpha and parallel beta sheets of 6 strands.<ref>''Protein: Alcohol dehydrogenase from Human (Homo sapiens), different isozymes''. SCOP. 2009. 1 March 2010 < http://web.archive.org/web/20060914235939/http://scop.berkeley.edu/data/scop.b.d.c.b.b.c.htmcop.berkeley.edu/data/scop.b.d.c.b.b.c.html></ref> |
| Line 48: | Line 48: | ||
[[image:Mechanism of adh.jpg|left|300px]] | [[image:Mechanism of adh.jpg|left|300px]] | ||
{{Clear}} | {{Clear}} | ||
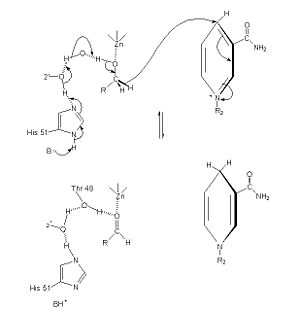
| - | The of alcohol dehydrogenase reaction is as follows: CH3CH2OH + NAD+ -> CH3COH (acetaldehyde) + NADH + H+ (Note: The reaction is actually reversible although the arrow does not show it) <ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> The step-wise reduction mechanism for ADH is shown on the left. In the mechanism, His 51 is deprotonated and activated by a base catalyst. This allows histidine to accept a proton from NAD, which also draws a proton Thr 48. As a result of the proton transfer, the Thr is prepared to accept a proton from the alcohol substrate. While Thr accepts the proton, there is also a hydride transfer to NAD. The whole process can be summarized as the oxidation of an alcohol to an aldehyde in concert with the transfer of a hydride to NAD.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref><ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> | + | The of alcohol dehydrogenase reaction is as follows: CH3CH2OH + NAD+ -> CH3COH (acetaldehyde) + NADH + H+ (Note: The reaction is actually reversible although the arrow does not show it) <ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> The step-wise reduction mechanism for ADH is shown on the left. In the mechanism, His 51 is deprotonated and activated by a base catalyst. This allows histidine to accept a proton from NAD, which also draws a proton Thr 48. As a result of the proton transfer, the Thr is prepared to accept a proton from the alcohol substrate. While Thr accepts the proton, there is also a hydride transfer to NAD. The whole process can be summarized as the oxidation of an alcohol to an aldehyde in concert with the transfer of a hydride to NAD.<ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref><ref>''Protein: Alcohol Dehydrogenase''. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm></ref> |
The Mechanism for alcohol dehydrogenase follows an random bisubstrate mechanism.<ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> In the mechanism, the NAD+ and alcohol bind to the enzyme, so that the enzyme is now attached to the two subtrates. While attached, the hydrogen is formally transferred from the alcohol to NAD, resulting in the products NADH and a ketone or aldehyde. The two products are then released, and the enzyme has catalyzed the reaction. | The Mechanism for alcohol dehydrogenase follows an random bisubstrate mechanism.<ref>Voet, et. al. ''Fundamentals of Biochemistry: 3rd Edition''. Hoboken: Wiley & Sons, Inc, 2008.</ref> In the mechanism, the NAD+ and alcohol bind to the enzyme, so that the enzyme is now attached to the two subtrates. While attached, the hydrogen is formally transferred from the alcohol to NAD, resulting in the products NADH and a ketone or aldehyde. The two products are then released, and the enzyme has catalyzed the reaction. | ||
Revision as of 15:02, 8 January 2017
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
3D Structures of Alcohol dehydrogenase
Updated on 08-January-2017
References
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 <http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm
- ↑ Protein: Alcohol dehydrogenase from Human (Homo sapiens), different isozymes. SCOP. 2009. 1 March 2010 < http://web.archive.org/web/20060914235939/http://scop.berkeley.edu/data/scop.b.d.c.b.b.c.htmcop.berkeley.edu/data/scop.b.d.c.b.b.c.html>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Bille V, Remacle J. Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose. Eur J Biochem. 1986 Oct 15;160(2):343-8. PMID:3769934
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Blomstrand R, Ostling-Wintzell H, Lof A, McMartin K, Tolf BR, Hedstrom KG. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: an approach to therapy against methanol poisoning. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3499-503. PMID:115004
- ↑ Alcohol Dehydrogenase. Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Alcohol Dehydrogenase.Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Goihberg E, Dym O, Tel-Or S, Levin I, Peretz M, Burstein Y. A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins. 2007 Jan 1;66(1):196-204. PMID:17063493 doi:10.1002/prot.21170
- ↑ Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution. Proteins. 2008 Feb 7;. PMID:18260103 doi:10.1002/prot.21946
- ↑ Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon L, Frolow F, Burstein Y. Biochemical and Structural Properties of Chimeras Constructed by Exchange of Cofactor-Binding Domains in Alcohol Dehydrogenases from Thermophilic and Mesophilic Microorganisms. Biochemistry. 2010 Feb 9. PMID:20102159 doi:10.1021/bi901730x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Joel L. Sussman, David Birrer