Fosamax (alendronate sodium)
From Proteopedia
| Line 29: | Line 29: | ||
</table> | </table> | ||
| - | Fosamax (Alendronate Sodium or Alendronic Acid) is a pharmaceutical drug administered | + | Fosamax (Alendronate Sodium or Alendronic Acid) is a pharmaceutical drug administered as an antiresorptive therapy agent for osteodegenerative diseases such as [https://en.wikipedia.org/wiki/Osteoporosis Osteoporosis], Paget's disease, and osteogenesis imperfecta<ref>https://www.pharmgkb.org/pathway/PA154423660</ref>. |
| + | Functioning as a nitrogen-containing, second generation [https://en.wikipedia.org/wiki/Bisphosphonate bisphosphonate], Fosamax binds to [https://en.wikipedia.org/wiki/Hydroxylapatite hydroxyapatite] in bone and promotes apoptosis in [https://en.wikipedia.org/wiki/Osteoclast osteoclasts] (cells specialized in skeletal breakdown), thereby delaying the degradation of bone tissue. While incorporated in bone matrix, alendronate is not pharmacologically active, thus, it must be continuously administered to suppress osteoclasts on newly formed resorption surfaces <ref>Fosamax: Uses, Dosage & Side Effects - Drugs.com. (2016, November 3).Fosamax: Uses, Dosage & Side Effects - Drugs.com. Retrieved November 14, 2016, from https://www.drugs.com/fosamax.html</ref>. | ||
== History <ref>Fosamax (alendronate sodium). Fosamax New FDA Drug Approval | CenterWatch. Merck. Retrieved November 15, 2016, from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/26/fosamax-alendronate-sodium</ref>== | == History <ref>Fosamax (alendronate sodium). Fosamax New FDA Drug Approval | CenterWatch. Merck. Retrieved November 15, 2016, from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/26/fosamax-alendronate-sodium</ref>== | ||
Revision as of 18:45, 1 December 2016
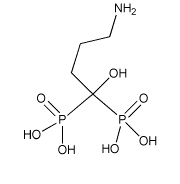
| Formula: | C4H13NO7P2 |
| Molar Mass: | 249.097 g/mol |
| INN: | Alendrotnic Acid |
| USAN: | Alendronate sodium |
| IUPAC: | 4-amino-1-hydroxybutylidene) bisphosphonic acid monosodium salt trihydrate |
Fosamax (Alendronate Sodium or Alendronic Acid) is a pharmaceutical drug administered as an antiresorptive therapy agent for osteodegenerative diseases such as Osteoporosis, Paget's disease, and osteogenesis imperfecta[1]. Functioning as a nitrogen-containing, second generation bisphosphonate, Fosamax binds to hydroxyapatite in bone and promotes apoptosis in osteoclasts (cells specialized in skeletal breakdown), thereby delaying the degradation of bone tissue. While incorporated in bone matrix, alendronate is not pharmacologically active, thus, it must be continuously administered to suppress osteoclasts on newly formed resorption surfaces [2].
Contents |
History [3]
Fosamax has been in use since September 29,1995 after gaining Food and Drug Administraion (FDA) approval. The drug obtained FDA clearance based on data from five clinical trials, lasting two years, involving 1,827 postmenopausal women with osteoporosis in 16 different countries. The trials showed a significant increase in bone mineral density at the spine, hip, and other sites. Overall, the drug reduced the number of women with new spinal fractures by 48%, the total number of new spinal fractures by 63%, and reduced overall height loss by 35%. Today, the drug remains in circulation in caplet and liquid forms and attainable at most pharmaceutical distribution centers with eligible prescription.
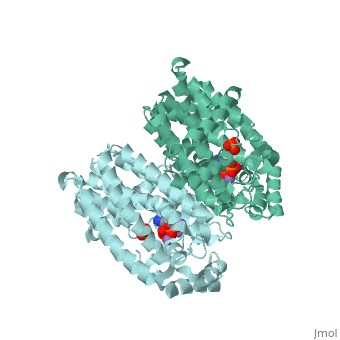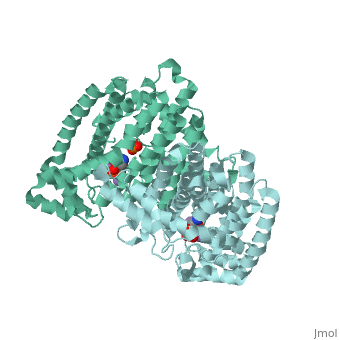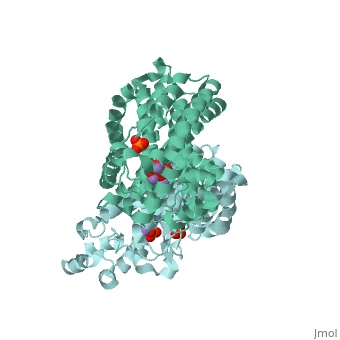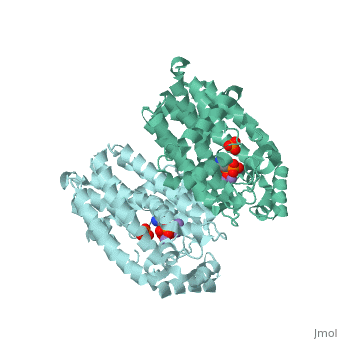
Structural Highlights [4]
| |||||||||||
Function
Functioning as a nitrogen-containing, second generation bisphosphonate, Fosamax binds to hydroxyapatite in bone and promotes apoptosis in osteoclasts (cells specialized in skeletal breakdown), thereby slowing the degradation of bone tissue[5]. Furthermore, alendronic acid binds to and inhibits the activity of farnesyl pyrophosphate synthase, an enzyme catalyzes a step in a biochemical pathway essential for homeostatic regulation of osteoclastic activity.
Mechanism
Fosamax inhibits farnesyl pyrophosphate synthase (FPS or FPPS), one of the enzymes found in the mevalonic acid pathway, which produces isoprenoid compounds that are essential for post-translational modification of small guanosine triphosphate (GTP)-binding proteins, such as Rho, Ras, and Rab. Inhibition of this process interferes with osteoclast function and survival [6][7]. The net negative charge of alendronic acid is thought to bind to three aspartate residues while also forming electrostatic interactions with Magnesium cofactors, binding in competition with other aminobisphosphates such as pamidronate and risedronate.
Side Effects [8]
Common side effects of Fosamax include gas, constipation, heartburn, diarrhea, bloating, nausea, vomiting, stomach pain, joint pain or swelling, swelling in your hands or feet, dizziness, headache, eye pain, back pain, or weakness. Serious side effects of Fosamax include severe pain (joints, bone, muscle, jaw, back or heartburn), chest pain, difficulty swallowing, bloody stools, eye pain, skin blisters, and swelling of the face, tongue, or throat.
References
- ↑ https://www.pharmgkb.org/pathway/PA154423660
- ↑ Fosamax: Uses, Dosage & Side Effects - Drugs.com. (2016, November 3).Fosamax: Uses, Dosage & Side Effects - Drugs.com. Retrieved November 14, 2016, from https://www.drugs.com/fosamax.html
- ↑ Fosamax (alendronate sodium). Fosamax New FDA Drug Approval | CenterWatch. Merck. Retrieved November 15, 2016, from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/26/fosamax-alendronate-sodium
- ↑ Protein Data Bank in Europe: Bringing Structure to Biology, from http://www.ebi.ac.uk/pdbe/entry/pdb/2F89
- ↑ Drake, Matthew T., Bart L. Clarke, and Sundeep Khosla. "Bisphosphonates: Mechanism of Action and Role in Clinical Practice." Mayo Clinic Proceedings 83, no. 9 (2008): 1032-045. doi:10.4065/83.9.1032.
- ↑ Alendronic acid. (2005, June 13), from http://www.drugbank.ca/drugs/DB00630#bond-15126
- ↑ Fisher, J. E., Rogers, M. J., Halasy, J. M., Luckman, S. P., Hughes, D. E., Masarachia, P. J., … Reszka, A. A. (1999). Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proceedings of the National Academy of Sciences, 96(1), 133–138. doi:10.1073/pnas.96.1.133, from http://www.pnas.org/content/96/1/133.full.pdf
- ↑ Common Side Effects of Fosamax (Alendronate Sodium) Drug Center - RxList. (2015, August 26). RxList. Retrieved November 14, 2016, from http://www.rxlist.com/fosamax-side-effects-drug-center.htm
Contributors and Editors
Floro, Alyssa Jewel D. Kidd, Justin M. Samady, Osna M.</font>