Carbidopa
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structure == | == Structure == | ||
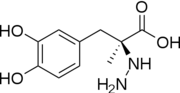
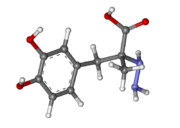
| - | [[Image:512px-Carbidopa.svg.png|thumb|right|2D Structure of Carbidopa]][[Image:800px-Carbidopa ball-and-stick.png|thumb|right|Ball and Stick Model of Carbidopa]]Carbidopa is an inhibitor of DDC. It is a partially water soluble, white, crystalline compound with a molecular weight of 226.232g/mol and a melting point of 208°C. Its empirical formula is | + | [[Image:512px-Carbidopa.svg.png|thumb|right|2D Structure of Carbidopa]][[Image:800px-Carbidopa ball-and-stick.png|thumb|right|Ball and Stick Model of Carbidopa]]Carbidopa is an inhibitor of DDC. It is a partially water soluble, white, crystalline compound with a molecular weight of 226.232g/mol and a melting point of 208°C. Its empirical formula is C<sub>10</sub>H<sub>14</sub>N<sub>2</sub>O<sub>4</sub>•H<sub>2</sub>O and its IUPAC name is (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid. Used in conjunction with Levadopa (L-DOPA), a precursor to dopamine, it increases concentrations of L-DOPA in the brain. Due to the fact Carbidopa cannot cross the blood–brain barrier, it inhibits only peripheral DDC thus preventing the conversion of L-DOPA to dopamine outside of neuronal cells. This greatly lessens the side effects caused by dopamine on the periphery, as well as increasing the concentration of L-DOPA and subsequently dopamine in the brain.<ref name="two">https://pubchem.ncbi.nlm.nih.gov/compound/carbidopa</ref> |
== Molecular Mechanism == | == Molecular Mechanism == | ||
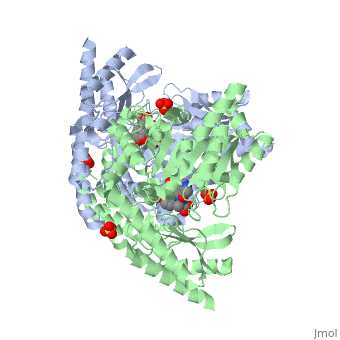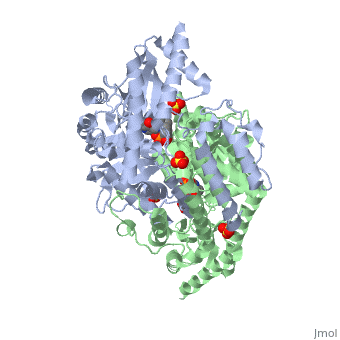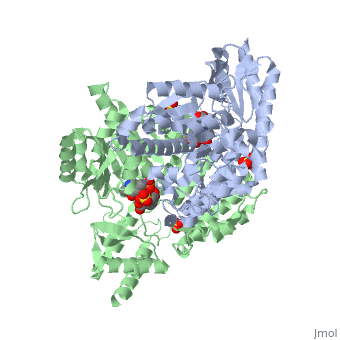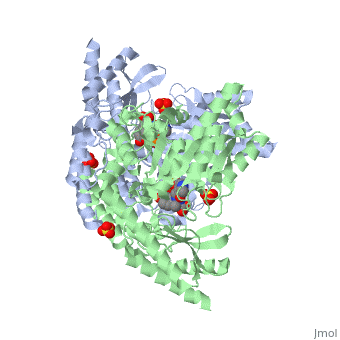
| - | The vitamin B6 (<scene name='pdbligand=PLP:PYRIDOXAL-5-PHOSPHATE'>PLP</scene>)-dependent enzyme DDC, an enzyme abundant in the nervous system as well as kidneys of humans, catalyzes the conversion of L-DOPA into dopamine.<ref name="three">PMID: 7651438</ref> The catalytically active form of DDC is a homodimer, a feature typical of this class of enzymes.<ref name= "four">PMID: 10673430</ref> DDCs active site is located between the two monomers but is mainly composed of residues from only one of the monomers. The cofactor PLP binds to Lys 303 through a Schiff base linkage and a salt bridge between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP, which acts as a strong electron sink capable of stabilizing the carbanionic intermediates produced by active DDC. Cofactor is further stabilized in the enzyme through a network of hydrogen bonds. Carbidopa works by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety and blocking the DDC <scene name='74/746001/Active_site_dopa_final/1'>active site</scene> residues Ile 101' and Phe 103' in the substrate binding pocket with its catechol ring.<ref name="five">doi:10.1038/nsb1101-963</ref> In addition, the 4' hydroxyl group of Carbidopas catechol ring hydrogen bonds with THR 82 and the carboxylate group binds to HIS 192, a highly-conserved residue in PLP-dependent decarboxylases.<ref name="six">PMID: 8889823</ref> Due to the fact that Carbidopa cannot cross the blood-brain barrier, its inhibiting effects only are displayed in the periphery. | + | The vitamin B6 (<scene name='pdbligand=PLP:PYRIDOXAL-5-PHOSPHATE'>PLP</scene>)-dependent enzyme DDC, an enzyme abundant in the nervous system as well as kidneys of humans, catalyzes the conversion of L-DOPA into dopamine.<ref name="three">PMID: 7651438</ref> The catalytically active form of DDC is a homodimer, a feature typical of this class of enzymes.<ref name= "four">PMID: 10673430</ref> DDCs active site is located between the two monomers but is mainly composed of residues from only one of the monomers. The cofactor <scene name='74/746001/Plp_binding/1'>PLP</scene> binds to Lys 303 through a Schiff base linkage and a salt bridge between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP, which acts as a strong electron sink capable of stabilizing the carbanionic intermediates produced by active DDC. Cofactor is further stabilized in the enzyme through a network of hydrogen bonds. Carbidopa works by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety and blocking the DDC <scene name='74/746001/Active_site_dopa_final/1'>active site</scene> residues Ile 101' and Phe 103' in the substrate binding pocket with its catechol ring.<ref name="five">doi:10.1038/nsb1101-963</ref> In addition, the 4' hydroxyl group of Carbidopas catechol ring hydrogen bonds with THR 82 and the carboxylate group binds to HIS 192, a highly-conserved residue in PLP-dependent decarboxylases.<ref name="six">PMID: 8889823</ref> Due to the fact that Carbidopa cannot cross the blood-brain barrier, its inhibiting effects only are displayed in the periphery. |
==Interactions== | ==Interactions== | ||
Current revision
Carbidoba ((2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid)
| |||||||||||
References
- ↑ Gilbert JA, Frederick LM, Ames MM. The aromatic-L-amino acid decarboxylase inhibitor carbidopa is selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells. Clin Cancer Res. 2000 Nov;6(11):4365-72. PMID:11106255
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/carbidopa
- ↑ Opacka-Juffry J, Brooks DJ. L-dihydroxyphenylalanine and its decarboxylase: new ideas on their neuroregulatory roles. Mov Disord. 1995 May;10(3):241-9. PMID:7651438 doi:http://dx.doi.org/10.1002/mds.870100302
- ↑ Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000 Jan 15;8(1):R1-6. PMID:10673430
- ↑ Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243 doi:http://dx.doi.org/10.1038/nsb1101-963
- ↑ Ishii S, Mizuguchi H, Nishino J, Hayashi H, Kagamiyama H. Functionally important residues of aromatic L-amino acid decarboxylase probed by sequence alignment and site-directed mutagenesis. J Biochem. 1996 Aug;120(2):369-76. PMID:8889823
- ↑ Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008 Sep 30;6(9):e236. doi: 10.1371/journal.pbio.0060236. PMID:18828673 doi:http://dx.doi.org/10.1371/journal.pbio.0060236
- ↑ 8.0 8.1 Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE. The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr Neuropharmacol. 2011 Jun;9(2):278-88. doi: 10.2174/157015911795596612. PMID:22131937 doi:http://dx.doi.org/10.2174/157015911795596612
- ↑ Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000 Mar 23;404(6776):394-8. PMID:10746727 doi:http://dx.doi.org/10.1038/35006074
- ↑ http://www.parkinson.org/understanding-parkinsons/treatment/Medications-for-Motor-Symptoms/Carbidopa-levodopa
- ↑ Fanali S, Pucci V, Sabbioni C, Raggi MA. Quality control of benserazide-levodopa and carbidopa-levodopa tablets by capillary zone electrophoresis. Electrophoresis. 2000 Jul;21(12):2432-7. PMID:10939456 doi:<2432::AID-ELPS2432>3.0.CO;2-E http://dx.doi.org/10.1002/1522-2683(20000701)21:12<2432::AID-ELPS2432>3.0.CO;2-E
- ↑ http://www.merck.com/product/home.html