Sandbox 45673
From Proteopedia
| Line 12: | Line 12: | ||
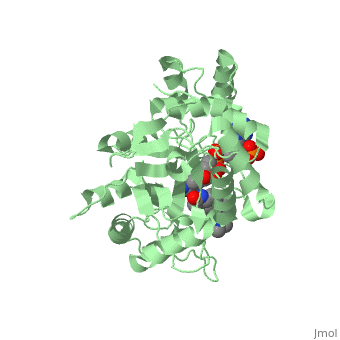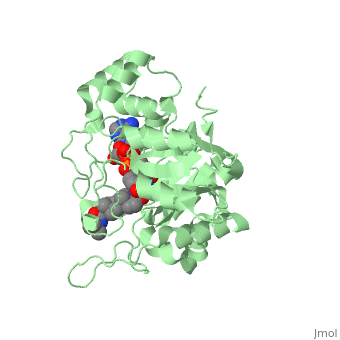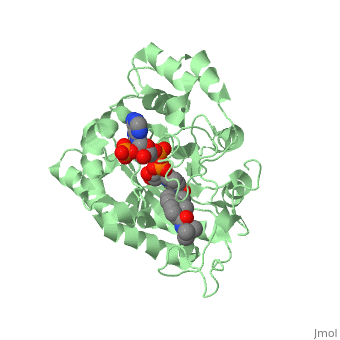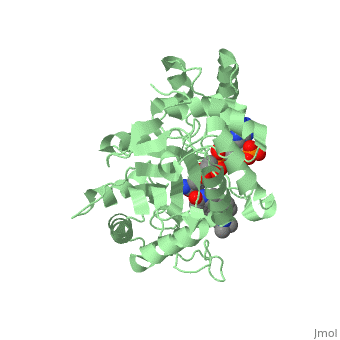
In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids. The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide has been added onto the carbon 17. The nitrogen present in the carboxamide has been with a tert-butyl group. | In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids. The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide has been added onto the carbon 17. The nitrogen present in the carboxamide has been with a tert-butyl group. | ||
| - | [[Image: | + | [[Image:Finasteride mechanism.png|left|430px]] |
==Mechanism== | ==Mechanism== | ||
Revision as of 21:56, 5 December 2016
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide
| |||||||||||
References
Allen, Helen. (2015, March). "Finasteride for prostate gland enlargement. Information. Patient.
Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t
Leyden, J., Dunlap, F., & Miller, B., et el. (1999, June). Finasteride in the treatment of men with frontal male pattern hair loss.
Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). The importance of dual 5α-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride.
Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm