We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 45673
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
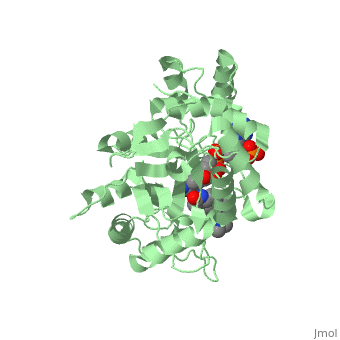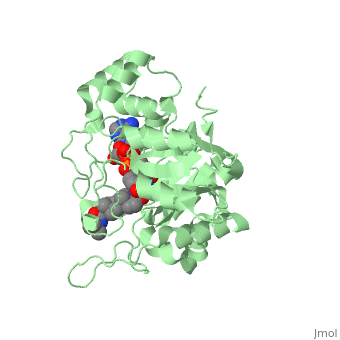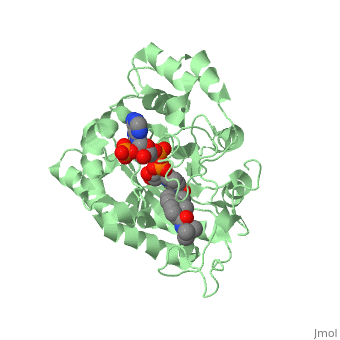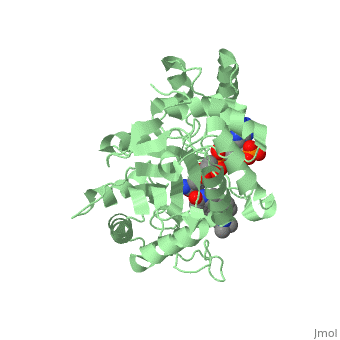
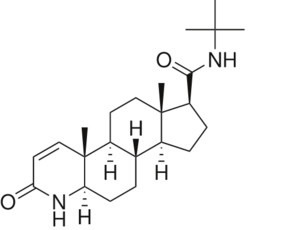
To see what Finasteride looks like unbound, click this link: <scene name='74/745970/Finasteride_unbound/1'>FIT</scene>. Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called a gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> Although a gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer. <ref name="eleven"> Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9 </ref> The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a ''tert''-butyl group bonded to the nitrogen atom, has been added onto the carbon 17. | To see what Finasteride looks like unbound, click this link: <scene name='74/745970/Finasteride_unbound/1'>FIT</scene>. Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called a gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> Although a gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer. <ref name="eleven"> Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9 </ref> The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a ''tert''-butyl group bonded to the nitrogen atom, has been added onto the carbon 17. | ||
| - | [[Image: Finasteride.PNG|thumb| | + | [[Image: Finasteride.PNG|thumb|300px|left|]] |
| + | {{Clear}} | ||
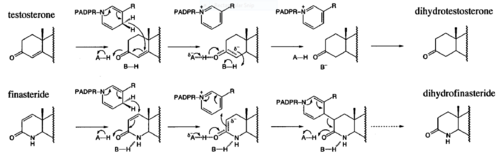
==Mechanism== | ==Mechanism== | ||
Revision as of 03:56, 6 December 2016
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide
| |||||||||||
References
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ 4.0 4.1 Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3
- ↑ Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9
- ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t