User:Camille Zumstein/Sandbox
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
''' Localisation and expression''': | ''' Localisation and expression''': | ||
| - | Calcineurin is a cytoplasmic protein. The catalytic subunit calcineurin A, posses two major isoforms called calcineurin Aα and Aβ. Calcineurin Aα is widely expressed among tissues especially in the Central Nervous System (CNS). Calcineurin Aβ is rather found in the lymphoid cells.Calcineurin Aβ is therefore involved in the mediation of the immune response | + | Calcineurin is a cytoplasmic protein. The catalytic subunit calcineurin A, posses two major isoforms called calcineurin Aα and Aβ. Calcineurin Aα is widely expressed among tissues especially in the Central Nervous System (CNS). Calcineurin Aβ is rather found in the lymphoid cells.Calcineurin Aβ is therefore involved in the mediation of the immune response (PMID:16888030). |
== Function == | == Function == | ||
| Line 14: | Line 14: | ||
<scene name='75/750223/Jw_caln_sec_strcutur/1'>Calcineurin</scene> is a heterodimeric Protein that consits of two subunits. They are called catalytic and regulatory subunit. Four molecules of Calcineurin form an asymmetric complex which leads to a total molecular weight of 370 kDa. | <scene name='75/750223/Jw_caln_sec_strcutur/1'>Calcineurin</scene> is a heterodimeric Protein that consits of two subunits. They are called catalytic and regulatory subunit. Four molecules of Calcineurin form an asymmetric complex which leads to a total molecular weight of 370 kDa. | ||
| - | The structure presented in this article is the catalytic subunit isoform of the serine/threonine-protein phosphatase 2B in [https://www.ncbi.nlm.nih.gov/UniGene/UGOrg.cgi?TAXID=10116 rattus norvegicus (rat)]. It consists of 521 | + | The structure presented in this article is the catalytic subunit isoform of the serine/threonine-protein phosphatase 2B in [https://www.ncbi.nlm.nih.gov/UniGene/UGOrg.cgi?TAXID=10116 rattus norvegicus (rat)]. It consists of 521 (http://www.uniprot.org/uniprot/P63329) aminoacids and has a molecular weight of 57 kDa (http://www.uniprot.org/uniprot/P63329). |
The calatytic subunit is subdivided into functional domains which are a <scene name='75/750223/Catalytique_domain_of_chain_a/1'>catalytic domain (here chain A is shown)</scene>, a <scene name='75/750223/Interact_dom_ca/1'>binding domain for the regulary subunit</scene>, a <scene name='75/750223/Calm_bind_dom_ca/1'>calmodulin binding domain </scene> and an <scene name='75/750223/Auto_inh_dom_ca/1'>autoinhibitory domain</scene>. | The calatytic subunit is subdivided into functional domains which are a <scene name='75/750223/Catalytique_domain_of_chain_a/1'>catalytic domain (here chain A is shown)</scene>, a <scene name='75/750223/Interact_dom_ca/1'>binding domain for the regulary subunit</scene>, a <scene name='75/750223/Calm_bind_dom_ca/1'>calmodulin binding domain </scene> and an <scene name='75/750223/Auto_inh_dom_ca/1'>autoinhibitory domain</scene>. | ||
| Line 24: | Line 24: | ||
'''Discovery of the structure:''' | '''Discovery of the structure:''' | ||
[[Image:4il1 multipercentile validation.png|thumb|upright=2|left]] | [[Image:4il1 multipercentile validation.png|thumb|upright=2|left]] | ||
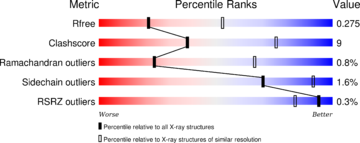
| - | The structure have been [https://www.ncbi.nlm.nih.gov/pubmed/24018048 published in the year 2013 by Qilu Ye et al.] | + | The structure have been [https://www.ncbi.nlm.nih.gov/pubmed/24018048 published in the year 2013 by Qilu Ye et al.] (PMID:24018048) in [http://www.sciencedirect.com/science/journal/08986568 ''Cellular Signaling'']. For their experiments they used [https://en.wikipedia.org/wiki/X-ray_crystallography#X-ray_diffraction x-ray diffraction]. The [http://www.wwpdb.org/validation/2016/XrayValidationReportHelp PDB validation] obtained a Resolutionof 3.0 Å, a free R-value of 0.273 and a work R-value of 0.241 (http://www.rcsb.org/pdb/explore/explore.do?structureId=4IL1). |
<br style="clear:both" /> | <br style="clear:both" /> | ||
| Line 43: | Line 43: | ||
== Principle of action == | == Principle of action == | ||
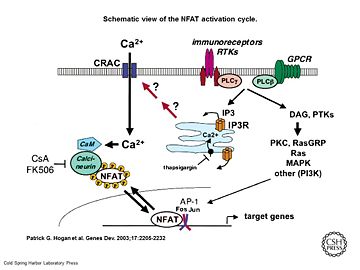
| - | [[Image:Genes Dev. 2003 Sep 17(18) 2205-32, Figure 1.jpg|thumb|upright=2|Schematic view of the NFAT activation cycle | + | [[Image:Genes Dev. 2003 Sep 17(18) 2205-32, Figure 1.jpg|thumb|upright=2|Schematic view of the NFAT activation cycle (PMID:12975316)]] |
| - | Calcineurin is activated by [http://www.ebi.ac.uk/interpro/potm/2003_3/Page_1.htm Calmodulin], a calcium-binding protein. Calmodulin interacts with the calmodulin-binding/regulatory region of Calcineurin. That binding leads to a conformational change in the autoinhibitory domain and remove it from the active site | + | Calcineurin is activated by [http://www.ebi.ac.uk/interpro/potm/2003_3/Page_1.htm Calmodulin], a calcium-binding protein. Calmodulin interacts with the calmodulin-binding/regulatory region of Calcineurin. That binding leads to a conformational change in the autoinhibitory domain and remove it from the active site (doi:10.1016/j.jmb.2011.11.008). |
| - | It has been reported that Calcineurin activates the transcription factor [https://de.wikipedia.org/wiki/NF-AT NFAT] by forming a complex and dephosporylation | + | It has been reported that Calcineurin activates the transcription factor [https://de.wikipedia.org/wiki/NF-AT NFAT] by forming a complex and dephosporylation (PMID:17502104). Following, the factor enters the nucleus and activates the expression of Interleukin-2. |
<br style="clear:both" /> | <br style="clear:both" /> | ||
| Line 53: | Line 53: | ||
== Binding Partners == | == Binding Partners == | ||
The main partners of interaction are [https://en.wikipedia.org/wiki/Calmodulin Calmodulin],NFATc1, NFATc2 and NFATc3. | The main partners of interaction are [https://en.wikipedia.org/wiki/Calmodulin Calmodulin],NFATc1, NFATc2 and NFATc3. | ||
| - | Many of the calcineurin substrates’ contain a PxIxIT motif. Among them, beside the phosphorylated forms of NFAT we can also mentioned; cAMP response element binding protein (CREB), PP1, microtubule-associated protein tau and glycogen synthase kinase-3 beta (GSK- 3) | + | Many of the calcineurin substrates’ contain a PxIxIT motif. Among them, beside the phosphorylated forms of NFAT we can also mentioned; cAMP response element binding protein (CREB), PP1, microtubule-associated protein tau and glycogen synthase kinase-3 beta (GSK- 3)(PMID: 17666045)(PMID: 22676853)(PMID: 14701880)(PMID: 7515479). |
| - | Calcineurin is inhibited by the immunosuppressive drugs tacrolismus (FK506) or cyclosporine A (CsA). CsA and FK506 conduct their therapeutic role thought binding to the [https://en.wikipedia.org/wiki/Immunophilins immunophilins] cyclophilin and FK506 binding protein (FK506BP) respectively. The complexes CsA-cyclophilin and FK506-FK506BP bind then to calcineurin in a calcium-dependent manner thus inhibiting its phosphatase activity. Therefore the addition of these drugs to lymphocytes T prevent NFAT translocation to the nucleus and the subsequent activation its target gene.That's why FK506 and CsA are use in the treatment of various immune-mediated diseases. However since calcineurin is is widely expressed in non-haemopoietic tissues like the kidney and the hearth, both drugs present a long term toxicity and can lead to deleterious effect to these | + | Calcineurin is inhibited by the immunosuppressive drugs tacrolismus (FK506) or cyclosporine A (CsA). CsA and FK506 conduct their therapeutic role thought binding to the [https://en.wikipedia.org/wiki/Immunophilins immunophilins] cyclophilin and FK506 binding protein (FK506BP) respectively. The complexes CsA-cyclophilin and FK506-FK506BP bind then to calcineurin in a calcium-dependent manner thus inhibiting its phosphatase activity. Therefore the addition of these drugs to lymphocytes T prevent NFAT translocation to the nucleus and the subsequent activation its target gene.That's why FK506 and CsA are use in the treatment of various immune-mediated diseases. However since calcineurin is is widely expressed in non-haemopoietic tissues like the kidney and the hearth, both drugs present a long term toxicity and can lead to deleterious effect to these Organs (PMID: 8811062), (http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus). |
'''Cofactors''': | '''Cofactors''': | ||
| - | Calcineurin belong to the family of [https://en.wikipedia.org/wiki/Metalloprotein metalloprotein]. To conduct its activity it requires the presence of Fe3+ and Zn2+ ions in the active site (one per subunit).Superoxide dismutase has been shown to protect calcineurin from inactivation by preventing Fe3+ from oxidation. Thus after activation of calcineurin by calmodulin, the AID is displaced from the catalytic core exposing Fe3+ to oxidation | + | Calcineurin belong to the family of [https://en.wikipedia.org/wiki/Metalloprotein metalloprotein]. To conduct its activity it requires the presence of Fe3+ and Zn2+ ions in the active site (one per subunit).Superoxide dismutase has been shown to protect calcineurin from inactivation by preventing Fe3+ from oxidation. Thus after activation of calcineurin by calmodulin, the AID is displaced from the catalytic core exposing Fe3+ to oxidation (PMID: 8837775)(Calmodulin and Signal Transduction (p184), Linda J. Van Eldik,D. Martin Watterson (1998)). |
| Line 67: | Line 67: | ||
== Related health defects == | == Related health defects == | ||
| - | Calcineurin hyperactivation thought dysregulation of the Ca2+ dynamic have been show to play a critical role in several diseases like Rheumatoid arthritis (RA), Schizophrenia ,Diabetes, Systemic Lupus Erythematosus as well as Alzheimer diseases | + | Calcineurin hyperactivation thought dysregulation of the Ca2+ dynamic have been show to play a critical role in several diseases like Rheumatoid arthritis (RA), Schizophrenia ,Diabetes, Systemic Lupus Erythematosus as well as Alzheimer diseases (http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus)(PMID: 12851457)(PMID: 16988714)(PMID: 20421909)(PMID: 22654726).In order to fight those health defects calmodulin inhibitors can be administrated. |
Revision as of 14:26, 14 January 2017
Structure Rat Calcineurin
| |||||||||||