User:Camille Zumstein/Sandbox
From Proteopedia
| Line 59: | Line 59: | ||
| - | + | <br style="clear:both" /> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 14:13, 23 January 2017
Structure Rat Calcineurin
is a serine/threonine phosphatase that is activated by calmodulin(CaM) in a calcium-dependant manner. Calcineurin is the only known calmodulin-activated protein phosphatase. Calcineurin is responsible mainly for dephosphorylating several member of the NFAT transcription factor family (including NFATc1 through NFATc4). Localisation and expression: Calcineurin is a cytoplasmic protein. The catalytic subunit calcineurin A, posses two major isoforms called calcineurin Aα and Aβ. Calcineurin Aα is widely expressed among tissues especially in the Central Nervous System (CNS). Calcineurin Aβ is rather found in the lymphoid cells.Calcineurin Aβ is therefore involved in the mediation of the immune response [1].
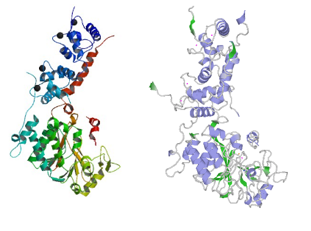
FunctionAfter activation by CaM, Calcineurin play a important role in multiple pathways. Calcineurin can modulates the immune response, act on the muscle formation and remodeling as well as on the neuronal plasticity and the cell death. Calcineurin is able to desphosphorylate a number of ion channels and thus modulate the basal excitability of neurons. Calcineurin has also an effect on the synaptic transmission since it affect the release of several neurotransmitters[2][3]. General structureis a heterodimeric Protein that consits of two subunits. They are called catalytic and regulatory subunit. Four molecules of Calcineurin form an asymmetric complex which leads to a total molecular weight of 370 kDa. The structure presented in this article is the catalytic subunit isoform of the serine/threonine-protein phosphatase 2B in rattus norvegicus (rat). It consists of 521 [1] aminoacids and has a molecular weight of 57 kDa [2]. The calatytic subunit is subdivided into functional domains which are a , a , a and an . The catalytic side includes a residue (green) at position 151 that acts as proton donor and metal binding sites. (shown in brown) binds at the position 118, 150, 199 and 281. Iron (blue) interacts at the positions 90, 92 and 118. are modified with a serine or a tyrosine. At position 2, a N-acetylserine has been found by similarity, as well as a nitrated tyrosine at position 224, and phosphoserine at position 469 and 492.
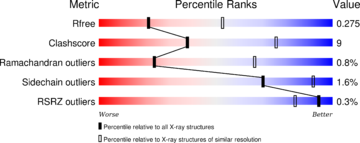
Discovery of the structure: The structure have been published in the year 2013 by Qilu Ye et al. [4] in Cellular Signaling. For their experiments they used x-ray diffraction. The PDB validation obtained a Resolutionof 3.0 Å, a free R-value of 0.273 and a work R-value of 0.241 [3].
secondary structure:  The secondary structure mostly includes helical structures Uniprot Besides, there are six turns reported in the structure of one chain.
Principle of action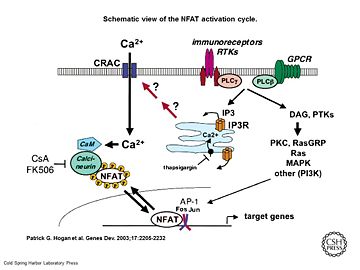 Schematic view of the NFAT activation cycle [5] As a response of receptor tyrosine kinase [4] activation as well as G protein-coupled receptor (GCPR) activation the Phospholipase C (PLC) catalyse the hydrolysis of PIP2 to IP3 and DAG. IP3 activates the Inositol 1,4,5-Trisphosphate Receptor and therby leads to an increasing amount of the second Messenger Ca2+ in the cytoplasma. Calcineurin is activated by Calmodulin, a calcium-binding protein. Calmodulin interacts with the calmodulin-binding/regulatory region of Calcineurin. That binding leads to a conformational change in the autoinhibitory domain and remove it from the active site (doi:10.1016/j.jmb.2011.11.008). It has been reported that Calcineurin activates the transcription factor NFAT by forming a complex and dephosporylation [6]. Following, the factor enters the nucleus and activates the expression of Interleukin-2.
Binding Partners
The main partners of interaction are Calmodulin,NFATc1, NFATc2 and NFATc3. Many of the calcineurin substrates’ contain a PxIxIT motif. Among them, beside the phosphorylated forms of NFAT we can also mentioned; cAMP response element binding protein (CREB), PP1, microtubule-associated protein tau and glycogen synthase kinase-3 beta (GSK- 3)[7][8][9][10]. is inhibited by the immunosuppressive drugs tacrolismus (FK506) or cyclosporine A (CsA). CsA and conduct their therapeutic role thought binding to the immunophilins cyclophilin and FK506 binding protein (FK506BP) respectively. The complexes CsA-cyclophilin and FK506-FK506BP bind then to calcineurin in a calcium-dependent manner thus inhibiting its phosphatase activity. Therefore the addition of these drugs to lymphocytes T prevent NFAT translocation to the nucleus and the subsequent activation its target gene.That's why FK506 and CsA are use in the treatment of various immune-mediated diseases. However since calcineurin is is widely expressed in non-haemopoietic tissues like the kidney and the hearth, both drugs present a long term toxicity and can lead to deleterious effect to these Organs [11], (http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus).
Cofactors: Calcineurin belong to the family of metalloprotein. To conduct its activity it requires the presence of Fe3+ and Zn2+ ions in the active site (one per subunit).Superoxide dismutase has been shown to protect calcineurin from inactivation by preventing Fe3+ from oxidation. Thus after activation of calcineurin by calmodulin, the AID is displaced from the catalytic core exposing Fe3+ to oxidation [12](Calmodulin and Signal Transduction (p184), Linda J. Van Eldik,D. Martin Watterson (1998)).
Related health defectsCalcineurin hyperactivation thought dysregulation of the Ca2+ dynamic have been show to play a critical role in several diseases like Rheumatoid arthritis (RA), Schizophrenia ,Diabetes, Systemic Lupus Erythematosus as well as Alzheimer diseases(AD) (http://www.uptodate.com/contents/pharmacology-of-cyclosporine-and-tacrolimus)[13][14][15]. Taking the example of AD which is a age-related memory dysfunction ; it it know that in older organism the brain is less plastic due to a dysregulation of Ca2+ dynamic. This in addition to the presence of oligomeric Aß is sufficient to explain an enhancement of CaN activity leading to severals symptoms like decreased neurotransmission , synaptic loss , neuroinflammation ...[16]
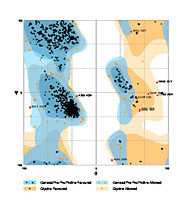
Therefore calmodulin inhibitors are potential alternatives against Alzheimer diseases. Human/Rat calcineurin comparisonHuman ([5]) and Rat calcineurin have the same function and global structure . The size and subunits of the linear structure (image)are the same, as well as the 3D structure.However, there are a few differences, such as the interactions proteins (image), and the secondary structures. cdire différence de structure secondaire et faire lien vers struct 3D pour feuillets etc
However
References
| ||||||||||||||||||