User:Estelle Metzger/Sandbox
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
For catalysis, the formation of a polar contact is essential. This polar contact takes place between Roco4 <scene name='75/751216/1055/2'>Lys1055</scene> from the beta3-strand and the <scene name='75/751216/1078/1'>Glu1078</scene> from the alphaC-helix. The amino acids Asp makes contact with all three ATP phosphates either directly or via coordination of a <scene name='75/751216/Mg/1'>magnesium ion</scene>. Moreover, the amino acid Phe makes hydrophobic contacts to the alphaC-helix and the HxD motif, and leads for the correct positioning of the DFG motif. <ref name="Bernd2"/> | For catalysis, the formation of a polar contact is essential. This polar contact takes place between Roco4 <scene name='75/751216/1055/2'>Lys1055</scene> from the beta3-strand and the <scene name='75/751216/1078/1'>Glu1078</scene> from the alphaC-helix. The amino acids Asp makes contact with all three ATP phosphates either directly or via coordination of a <scene name='75/751216/Mg/1'>magnesium ion</scene>. Moreover, the amino acid Phe makes hydrophobic contacts to the alphaC-helix and the HxD motif, and leads for the correct positioning of the DFG motif. <ref name="Bernd2"/> | ||
| - | Roco4 has two conformation, an active conformation and an inactive conformation. These conformations depend of the conformation of the DFG motif : a DFG-in (active) and a DFG-out (inactive) conformation. Therefore, in the structure of active Roco4 kinase, the activation loop is visible and ordered. In contrast, in the structure of inactive Roco4 kinase, the activation loop is not visible.<ref name="Huse"> Morgan Huse, John Kuriyan. The Conformational Plasticity of Protein Kinases. Cell. May 2002. doi: 10.1016/S0092-8674(02)00741-9</ref> <ref name="Taylor">doi: 10.1016/j.tibs.2010.09.006</ref> | + | Roco4 has two conformation, an active conformation and an inactive conformation. These conformations depend of the conformation of the DFG motif : a DFG-in (active) and a DFG-out (inactive) conformation. Therefore, in the structure of active Roco4 kinase, the activation loop is visible and ordered. In contrast, in the structure of inactive Roco4 kinase, the activation loop is not visible.<ref name="Huse"> Morgan Huse, John Kuriyan. The Conformational Plasticity of Protein Kinases. Cell. May 2002.|doi: 10.1016/S0092-8674(02)00741-9</ref> <ref name="Taylor">doi: 10.1016/j.tibs.2010.09.006</ref> |
In most kinases, there is a mechanism to switch from an inactive to an active state. | In most kinases, there is a mechanism to switch from an inactive to an active state. | ||
Revision as of 08:19, 27 January 2017
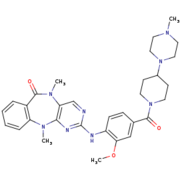
Humanized Roco4 bound to LRRK2-IN-1
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Gilsbach BK, Messias AC, Ito G, Sattler M, Alessi DR, Wittinghofer A, Kortholt A. Structural Characterization of LRRK2 Inhibitors. J Med Chem. 2015 May 1. PMID:25897865 doi:http://dx.doi.org/10.1021/jm5018779
- ↑ 2.0 2.1 2.2 2.3 2.4 Gilsbach BK, Kortholt A. Structural biology of the LRRK2 GTPase and kinase domains: implications for regulation. Front Mol Neurosci. 2014 May 5;7:32. doi: 10.3389/fnmol.2014.00032. eCollection, 2014. PMID:24847205 doi:http://dx.doi.org/10.3389/fnmol.2014.00032
- ↑ 3.0 3.1 Morgan Huse, John Kuriyan. The Conformational Plasticity of Protein Kinases. Cell. May 2002.|doi: 10.1016/S0092-8674(02)00741-9
- ↑ 4.0 4.1 Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci. 2011 Feb;36(2):65-77. doi: 10.1016/j.tibs.2010.09.006. Epub, 2010 Oct 23. PMID:20971646 doi:10.1016/j.tibs.2010.09.006
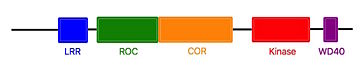
- ↑ 5.0 5.1 UniProtKB - Q5S007 (LRRK2_HUMAN), Retrieved on January 27th 2017.