We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Ophelie Lefort/Sandbox
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
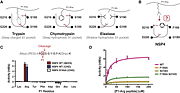
Recruitement of neutrophils to the site of inflammation is the earliest defense reaction against pathogens. NSP4 is stored in the azurophilic granules <ref>PMID:23904161</ref> which is a compartment of neutrophils. In response to neutrophil activation, NSP4 is released into the pericellular environment where microbes are after their phagocytosis. The serine protease 54 not only kill pathogens but also regulate the activity of immune mediators such as chemokines and lymphocytes. <ref>NSP4, an elastase-related protease in human neutrophils with arginine specificity | Recruitement of neutrophils to the site of inflammation is the earliest defense reaction against pathogens. NSP4 is stored in the azurophilic granules <ref>PMID:23904161</ref> which is a compartment of neutrophils. In response to neutrophil activation, NSP4 is released into the pericellular environment where microbes are after their phagocytosis. The serine protease 54 not only kill pathogens but also regulate the activity of immune mediators such as chemokines and lymphocytes. <ref>NSP4, an elastase-related protease in human neutrophils with arginine specificity | ||
| - | Natascha C. Perera,a Oliver Schilling,b Heike Kittel,a Walter Back,c Elisabeth Kremmer,d and Dieter E. Jenne doi:10.1073/pnas.1200470109</ref> NSP4 alterates chemokines by proteolitically cleaving N-terminus of the chemokine.<ref>PMC:3016231</ref> Furthermore, the caspase-like activity of PRSS57 can activate lymphocytes and the adaptive immune response. However, although NSP4 can inactivate some inflammatory processes, the neutrophil serine protease generally promotes than inhibits the inflammatory response. | + | Natascha C. Perera,a Oliver Schilling,b Heike Kittel,a Walter Back,c Elisabeth Kremmer,d and Dieter E. Jenne doi: 10.1073/pnas.1200470109</ref> NSP4 alterates chemokines by proteolitically cleaving N-terminus of the chemokine.<ref>PMC:3016231</ref> Furthermore, the caspase-like activity of PRSS57 can activate lymphocytes and the adaptive immune response. However, although NSP4 can inactivate some inflammatory processes, the neutrophil serine protease generally promotes than inhibits the inflammatory response. |
Revision as of 12:29, 27 January 2017
Neutrophil Serine 4 or Serine Protease 57 (4Q7X)
| |||||||||||
References
[3] NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity, NCBI
[4] NSP4, an elastase-related protease in human neutrophils with arginine specificity, NCBI
[5] Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response, NCBI
[6] Role of neutrophils in innate immunity: a systems biology-level approach, NCBI
[7] Neutrophils: Their Role in Innate and Adaptive Immunity, NCBI
[8] Neutrophil extracellular traps kill bacteria, NCBI
[9] Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response, NCBI
- ↑ Perera NC, Wiesmuller KH, Larsen MT, Schacher B, Eickholz P, Borregaard N, Jenne DE. NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity. J Immunol. 2013 Sep 1;191(5):2700-7. doi: 10.4049/jimmunol.1301293. Epub 2013 Jul, 31. PMID:23904161 doi:http://dx.doi.org/10.4049/jimmunol.1301293
- ↑ NSP4, an elastase-related protease in human neutrophils with arginine specificity Natascha C. Perera,a Oliver Schilling,b Heike Kittel,a Walter Back,c Elisabeth Kremmer,d and Dieter E. Jenne doi: 10.1073/pnas.1200470109
- ↑ PMC:3016231
- ↑ S.. Jack Lin, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, Daniel Kirchhofer Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease DOI: http://dx.doi.org/10.1016/j.str.2014.07.008
- ↑ Natascha C. Perera, Karl-Heinz Wiesmüller, Maria Torp Larsen, Beate Schacher, Peter Eickholz, Niels Borregaard and Dieter E. Jenne NSP4 Is Stored in Azurophil Granules and Released by Activated Neutrophils as Active Endoprotease with Restricted Specificity DOI: https://doi.org/10.4049/jimmunol.1301293
- ↑ Lin, S. Jack, Ken C. Dong, Charles Eigenbrot, Menno van Lookeren Campagne, and Daniel Kirchhofer. “Structures of Neutrophil Serine Protease 4 Reveal an Unusual Mechanism of Substrate Recognition by a Trypsin-Fold Protease.” Structure 22, no. 9 (September 2, 2014): 1333–40.[1]
- ↑ Perera, Natascha C., Karl-Heinz Wiesmüller, Maria Torp Larsen, Beate Schacher, Peter Eickholz, Niels Borregaard, and Dieter E. Jenne. “NSP4 Is Stored in Azurophil Granules and Released by Activated Neutrophils as Active Endoprotease with Restricted Specificity.” The Journal of Immunology 191, no. 5 (September 1, 2013) [2]