We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Elizeu/sandbox/citocromo c
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
''Streptococcus pneumoniae'', also widely known as pneumococcus, is a Gram positive coccus with a diameter of 0.5 to 1 μm. As a member of the genus ''Streptococcus'' it typically forms diplococci and can live both under aerobic or anaerobic conditions. In healthy carriers it resides in the nasopharynx <ref name="ref4">PMID:4366526</ref>. However, the bacterium may become pathogenic in elderly and [https://en.wikipedia.org/wiki/Immunodeficiency immunocompromised] people as well as in children due to a weaker immune system. In fact, ''S. pneumoniae'' is the most frequent opportunistic pathogen worldwide and causes many types of diseases including [https://en.wikipedia.org/wiki/Otitis_media otitis media], [https://en.wikipedia.org/wiki/Meningitis meningitis], [https://en.wikipedia.org/wiki/Sepsis sepsis] and [https://en.wikipedia.org/wiki/Pneumonia pneumonia] <ref>PMID:26379256</ref>. The genome of ''S. pneumoniae'' is a closed, circular DNA structure that contains between 2.0 and 2.1 million base pairs <ref name="ref4" />. | ''Streptococcus pneumoniae'', also widely known as pneumococcus, is a Gram positive coccus with a diameter of 0.5 to 1 μm. As a member of the genus ''Streptococcus'' it typically forms diplococci and can live both under aerobic or anaerobic conditions. In healthy carriers it resides in the nasopharynx <ref name="ref4">PMID:4366526</ref>. However, the bacterium may become pathogenic in elderly and [https://en.wikipedia.org/wiki/Immunodeficiency immunocompromised] people as well as in children due to a weaker immune system. In fact, ''S. pneumoniae'' is the most frequent opportunistic pathogen worldwide and causes many types of diseases including [https://en.wikipedia.org/wiki/Otitis_media otitis media], [https://en.wikipedia.org/wiki/Meningitis meningitis], [https://en.wikipedia.org/wiki/Sepsis sepsis] and [https://en.wikipedia.org/wiki/Pneumonia pneumonia] <ref>PMID:26379256</ref>. The genome of ''S. pneumoniae'' is a closed, circular DNA structure that contains between 2.0 and 2.1 million base pairs <ref name="ref4" />. | ||
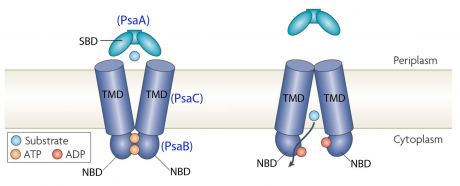
| + | [[Image:PsaA dans ABC transporteur.jpg |500px|thumb| Structure of ABC-transporter <ref>http://www.latrobe.edu.au/biochemistry-and-genetics/research/maher/psabca-manganese-uptake-by-streptococcus-pneumoniae</ref>]] | ||
| - | |||
| - | === PsaA protein === | ||
| - | |||
| - | Zinc in excess has a significant toxicity to bacteria because it is an important innate defense mechanism. There are many Zinc molecules in the human body. Manganese is important for the virulence, growth and proliferation of ''Streptococcus pneumoniae''. Zinc could compete for Manganese binding. However Manganese has more affinity for PsaA than Zinc but Zinc is not transported by the ABC-transporter. Zinc competition reduces intracellular Manganese resulting in up-regulation of PsaBCA expression. <ref>PMID:22072971</ref> | ||
| - | |||
| - | [[Image:PsaA dans ABC transporteur.jpg |500px|thumb| Structure of ABC-transporter <ref>http://www.latrobe.edu.au/biochemistry-and-genetics/research/maher/psabca-manganese-uptake-by-streptococcus-pneumoniae</ref>]] | ||
===ABC transporter=== | ===ABC transporter=== | ||
| Line 29: | Line 24: | ||
In the case of [https://www.wikigenes.org/e/gene/e/2717000.html PsaA], the ABC transporter is composed of an ATP-binding protein (''PsaB''), an integral membrane protein (''PsaC'') and PsaA itself as a [https://en.wikipedia.org/wiki/Lipoprotein lipoprotein] that possess a metal-ion binding site. This confers the protein to bind divalent metal-ions, however, with a preference for Mn<sup>2+</sup> <ref>PMID:18340341</ref>. | In the case of [https://www.wikigenes.org/e/gene/e/2717000.html PsaA], the ABC transporter is composed of an ATP-binding protein (''PsaB''), an integral membrane protein (''PsaC'') and PsaA itself as a [https://en.wikipedia.org/wiki/Lipoprotein lipoprotein] that possess a metal-ion binding site. This confers the protein to bind divalent metal-ions, however, with a preference for Mn<sup>2+</sup> <ref>PMID:18340341</ref>. | ||
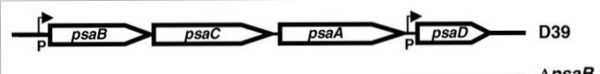
| - | The genes that encode for the components of the ABC transport system are ''psaA'', ''psaB'' and ''psaC'', respectively. They are organized consecutively in the psaBCA operon, together with ''psaD'', the gene encoding for a [thiol peroxidase] (see below). <ref name="ref3">PMID:517531</ref>.[[Image:operon psa.jpg | | + | The genes that encode for the components of the ABC transport system are ''psaA'', ''psaB'' and ''psaC'', respectively. They are organized consecutively in the psaBCA operon, together with ''psaD'', the gene encoding for a [[thiol peroxidase]] (see below). <ref name="ref3">PMID:517531</ref>. |
| + | |||
| + | [[Image:operon psa.jpg |600px|center| thumb| PsaBCA operon <ref>PMID:15255900</ref>]] | ||
| + | === Binding of zinc === | ||
| - | + | Zinc in excess has a significant toxicity to bacteria because it is an important innate defense mechanism. There are many zinc molecules in the human body which compete with manganese for PsaA binding. However manganese has a higher affinity for PsaA while zinc is not transported by the ABC-transporter. Nevertheless, the competition by zinc reduces intracellular manganese concentrations, resulting in the up-regulation of PsaBCA operon expression. <ref>PMID:22072971</ref> | |
| Line 45: | Line 43: | ||
[[Image:structure secondaire.jpg |1000 px|center| thumb| Secondary structure of PsaA <ref name="ref5" />]] | [[Image:structure secondaire.jpg |1000 px|center| thumb| Secondary structure of PsaA <ref name="ref5" />]] | ||
| - | |||
| - | |||
| - | |||
| Line 53: | Line 48: | ||
We can see the <scene name='55/559112/3ztt/1'>3D structure of PsaA with manganese</scene>. Manganese ions are shown as | We can see the <scene name='55/559112/3ztt/1'>3D structure of PsaA with manganese</scene>. Manganese ions are shown as | ||
<scene name='55/559112/3ztt_purple_sphere/1'>purple spheres</scene>. | <scene name='55/559112/3ztt_purple_sphere/1'>purple spheres</scene>. | ||
| + | {| | ||
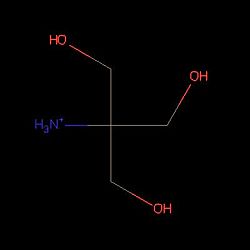
| + | | [[Image:molec.jpg|left|300px | thumb| 3D structure of PsaA with 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL (tris) and cadmium <ref>http://www.ebi.ac.uk/pdbe/entry/pdb/4UTO</ref>]] | ||
| + | | [[Image:showNew.jpg| 250px| thumb| Structure of Tris PsaA needs to be linked to this molecule to be in an open state]] | ||
| + | |} | ||
| - | [[Image:molec.jpg|left|300px | thumb| "3D structure of PsaA with 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL (tris) and cadmium<ref>http://www.ebi.ac.uk/pdbe/entry/pdb/4UTO</ref> | ||
| - | "]] | ||
| - | [[Image:showNew.jpg| 250px| thumb| "Structure of Tris | ||
| - | PsaA needs to be linked to this molecule to be in an open state"]] | ||
| - | |||
| - | |||
| - | This image shows the metal binding site in more detail, with the MntC residues, manganese (purple) and zinc (orange). We can see that Manganese interacts with Histine residues, Aspartique acid residues and Glutamique acid residues. | ||
| - | The metal binding site is formed by the sidechains of residues His67, His139, Glu205, and Asp280. This site has tetrahedral coordination geometry. The amino acids His67 andHis139 interact with the metal via Nε2 nitrogen atoms. However, the carboxylate sidechains of Glu205 and Asp280 interact with the metal via their Oε1 and Oδ2 atoms. The atomic distance between His67 Nε2 and the metal is 1.99 Ắ while it is 2,01 Ắ for His 139 Nε2. It is 2,04 Ắ for Glu205 Oε1 and 2,02 Ắ for Asp280 Oδ2. The Oδ1 atom of Asp137 can form a hydrogen bond with Nδ1 of His139. Oδ1 atom of Asp65 can also form a hydrogen bond with His67 N. <ref>PMID:9862808</ref> | ||
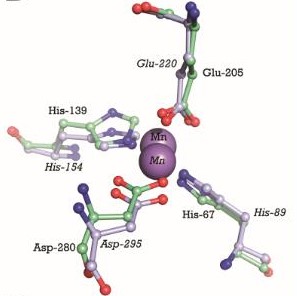
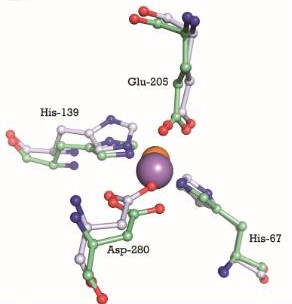
| + | The metal binding site is formed by the side chains of residues His67, His139, Glu205, and Asp280. This site has a tetrahedral coordination geometry. The amino acids His67 and His139 interact with the metal via Nε2 nitrogen atoms while the carboxylate side chains of Glu205 and Asp280 interact with the metal via their Oε1 and Oδ2 atoms. The atomic distance between His67 Nε2 and the metal is 1.99 Ắ while it is 2,01 Ắ for His 139 Nε2. It is 2,04 Ắ for Glu205 Oε1 and 2,02 Ắ for Asp280 Oδ2. The Oδ1 atom of Asp137 can form a hydrogen bond with Nδ1 of His139. Oδ1 atom of Asp65 can also form a hydrogen bond with His67 N. These interactions render PsaA its specificity to bind Mn<sup>2+</sup>, but also allows the binding for Zn<sup>2+</sup> <ref>PMID:9862808</ref>. | ||
Cadmium uptake reduces the millimolar cellular accumulation of manganese and zinc, and thereby increases sensitivity to oxidative stress <ref>http://www.nature.com/articles/ncomms7418</ref>. | Cadmium uptake reduces the millimolar cellular accumulation of manganese and zinc, and thereby increases sensitivity to oxidative stress <ref>http://www.nature.com/articles/ncomms7418</ref>. | ||
| Line 73: | Line 65: | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 116: | Line 104: | ||
-[https://www.wikigenes.org/e/gene/e/934852.html adcB], a zinc ABC transporter permease | -[https://www.wikigenes.org/e/gene/e/934852.html adcB], a zinc ABC transporter permease | ||
| + | |||
| + | |||
== Disease == | == Disease == | ||
=== Otitis media=== | === Otitis media=== | ||
PsaA has been recognized to be involved in the adherence and virulence mechanisms of otitis media. ''Streptococcus pneumoniae'' is one of the main agents causing bacterial acute otitis media, directly or as complication of a viral upper respiratory tract infection. This disease is a highly prevalent pediatric disease worldwide. Hearing loss is a common problem associated with this disease. ''Streptococcus pneumoniae'' in middle ear can be resistant to penicillin and cephalosporin<ref>https://www.google.com/patents/US20130078254</ref> | PsaA has been recognized to be involved in the adherence and virulence mechanisms of otitis media. ''Streptococcus pneumoniae'' is one of the main agents causing bacterial acute otitis media, directly or as complication of a viral upper respiratory tract infection. This disease is a highly prevalent pediatric disease worldwide. Hearing loss is a common problem associated with this disease. ''Streptococcus pneumoniae'' in middle ear can be resistant to penicillin and cephalosporin<ref>https://www.google.com/patents/US20130078254</ref> | ||
| + | |||
=== Pneumonia === | === Pneumonia === | ||
PsaA protein is an indirect [https://en.wikipedia.org/wiki/Bacterial_adhesin adhesin] which is involved in colonization of the nasopharyngeal mucosal. Moreover, alveolar pneumonia is caused by the spread of ''Streptococcus pneumoniae'' from nasopharynx. Therefore PsaA protein is involved in infection of pulmonary parenchyma by ''Streptococcus pneumoniae''. | PsaA protein is an indirect [https://en.wikipedia.org/wiki/Bacterial_adhesin adhesin] which is involved in colonization of the nasopharyngeal mucosal. Moreover, alveolar pneumonia is caused by the spread of ''Streptococcus pneumoniae'' from nasopharynx. Therefore PsaA protein is involved in infection of pulmonary parenchyma by ''Streptococcus pneumoniae''. | ||
Sometimes, ''Streptococcus pneumoniae'' pass into the blood and causes a bacteremia besides pneumonia. | Sometimes, ''Streptococcus pneumoniae'' pass into the blood and causes a bacteremia besides pneumonia. | ||
| + | |||
| + | |||
== Application in Biotechnology == | == Application in Biotechnology == | ||
PsaA is being actively evaluated as a component of a vaccin in formulations composed of pneumococcal common proteins. PsaA is a component of a vaccin because this protein is immunogenic and stimulates an increase in [https://en.wikipedia.org/wiki/Antibody antibody] production when the nasopharynx is naturally colonized. PsaA has been expressed as an ''E.coli'' recombinant protein, purified, and evaluated in a phase one clinical trial. | PsaA is being actively evaluated as a component of a vaccin in formulations composed of pneumococcal common proteins. PsaA is a component of a vaccin because this protein is immunogenic and stimulates an increase in [https://en.wikipedia.org/wiki/Antibody antibody] production when the nasopharynx is naturally colonized. PsaA has been expressed as an ''E.coli'' recombinant protein, purified, and evaluated in a phase one clinical trial. | ||
Progress in vaccine development is most advanced for ''Streptococcus pneumoniae''. Indeed, there is a seven-valent capsular-conjugate vaccine, [https://en.wikipedia.org/wiki/Pneumococcal_conjugate_vaccine PREVNAR®] but it is rather non efficient for otitis media.<ref>PMID:11176564</ref> PsaA has been shown to be an effective vaccine in animal models at preventing Streptococcus pneumoniae infection. | Progress in vaccine development is most advanced for ''Streptococcus pneumoniae''. Indeed, there is a seven-valent capsular-conjugate vaccine, [https://en.wikipedia.org/wiki/Pneumococcal_conjugate_vaccine PREVNAR®] but it is rather non efficient for otitis media.<ref>PMID:11176564</ref> PsaA has been shown to be an effective vaccine in animal models at preventing Streptococcus pneumoniae infection. | ||
| + | |||
| + | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 22:53, 27 January 2017
NCBI Accession: P42363.1
Uniprot Accesion: POA4G2
PDB ID: 3ZK7
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Julie Langlois, Atena Farhangian, Rebecca Holstein, Elizabeth A. Dunlap, Katherine Reynolds, Elizeu Santos, Noam Gonen, Anna Lohning, Idan Ben-Nachum, Brian Ochoa, Shai Biran, Gauri Misra, Shira Weingarten-Gabbay, Keni Vidilaseris, Jamie Costa, Abhinav Mittal, Urs Leisinger, Madison Walberry, Edmond R Atalla, Brett M. Thumm, Brooke Fenn, Joel L. Sussman, Mati Cohen, Vesta Nwankwo, Dotan Shaniv, Gulalai Shah