Sandbox Reserved 1067
From Proteopedia
| Line 10: | Line 10: | ||
== Electrostatic Interactions == | == Electrostatic Interactions == | ||
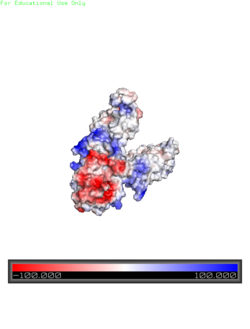
| - | + | Charge distribution along the exterior surface of the protein is primarily neutral for the trans-membrane domains, but transitions to positive near the location of the charge interlock and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation. Binding sites A, B, and C, as well as the C-terminus domains of both monomers, all possess a high negative charge relative to the other charges present, facilitating the binding and releasing of Zn<sup>2+</sup> ions. The two C-terminus domains are held together by the charge interlock and hydrophobic interactions of the trans-membrane domains despite their electrostatic repulsion. Upon the release of Zn<sup>2+</sup> ions, the C-terminus domains undergo electronegativity alterations, forcing the two domains apart. | |
== Conformation Changes == | == Conformation Changes == | ||
Revision as of 17:45, 17 March 2017
|
Contents |
Zinc Transporter Yiip
Structure
Helix Orientation
Salt Bridge
Electrostatic Interactions
Charge distribution along the exterior surface of the protein is primarily neutral for the trans-membrane domains, but transitions to positive near the location of the charge interlock and interior side of the cell membrane. This positive section is characteristic of trans-membrane proteins as a means of achieving proper orientation. Binding sites A, B, and C, as well as the C-terminus domains of both monomers, all possess a high negative charge relative to the other charges present, facilitating the binding and releasing of Zn2+ ions. The two C-terminus domains are held together by the charge interlock and hydrophobic interactions of the trans-membrane domains despite their electrostatic repulsion. Upon the release of Zn2+ ions, the C-terminus domains undergo electronegativity alterations, forcing the two domains apart.
Conformation Changes
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>