Carboxylesterase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | + | ||
<StructureSection load='4ab1' size='350' side='right' caption='Human carboxylesterase 1 (PDB code [[4ab1]]).' scene='Journal:Acta_Cryst_F:1/Cv/2'> | <StructureSection load='4ab1' size='350' side='right' caption='Human carboxylesterase 1 (PDB code [[4ab1]]).' scene='Journal:Acta_Cryst_F:1/Cv/2'> | ||
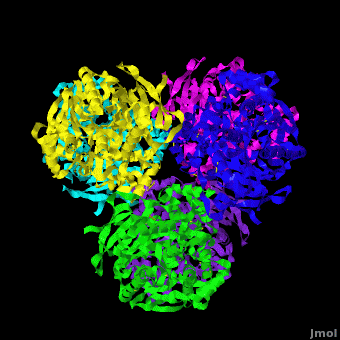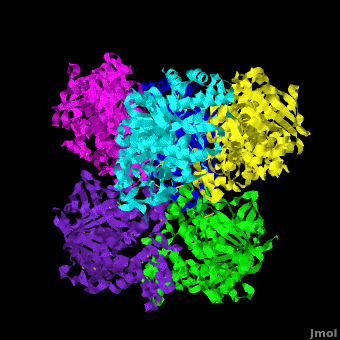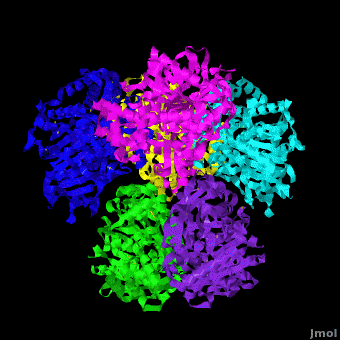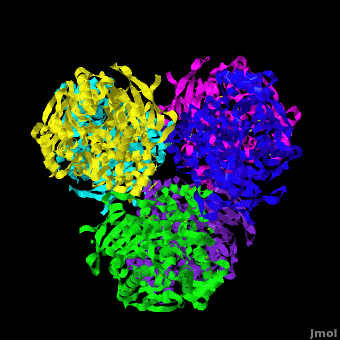
'''Carboxylesterase''' (CE) catalyzes the conversion of a wide variety carboxylic esters to alcohol and carboxylate. The catalytic triad of CE involves serine, glutamic acid or aspartic acid and histidine. Human CE1 (hCE1) is involved in drug metabolism and activation. It catalyzes the hydrolysis of heroin and cocaine. <br /> | '''Carboxylesterase''' (CE) catalyzes the conversion of a wide variety carboxylic esters to alcohol and carboxylate. The catalytic triad of CE involves serine, glutamic acid or aspartic acid and histidine. Human CE1 (hCE1) is involved in drug metabolism and activation. It catalyzes the hydrolysis of heroin and cocaine. <br /> | ||
Revision as of 05:58, 28 March 2017
| |||||||||||
3D structures of Carboxylesterase
Updated on 28-March-2017
References
- ↑ Greenblatt HM, Otto TC, Kirkpatrick MG, Kovaleva E, Brown S, Buchman G, Cerasoli DM, Sussman JL. Structure of recombinant human carboxylesterase 1 isolated from whole cabbage looper larvae. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Mar 1;68(Pt 3):269-72., Epub 2012 Feb 15. PMID:22442219 doi:10.1107/S1744309112003326