We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox1996
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='1stp' size='340' side='right' caption='The structure of chlorothizade (PDB code 3Ik6)' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='The structure of chlorothizade (PDB code 3Ik6)' scene=''> | ||
| + | == Structure == | ||
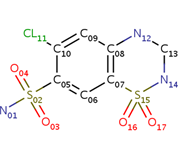
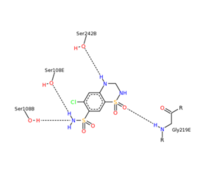
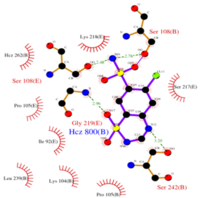
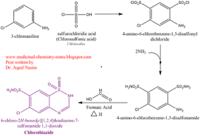
| - | + | Chlorothiazide is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its chemical formula is C7H8ClN3O4S2 and its molecular weight is of 298Da. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celcius, a solubility of 60 mg/m in DMSO and less than 1 mg/ml in water, and appears a white crystalline powder. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms. Cholorothiazide was determined when bound to glutamate receptor 2 through hydrogen bonding between the nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E. Synthesis of chlorothiazide occurs through the reaction between 3 -chloroaniline, chlorosulfonic acid, and ammonia; and its catalyzed by fomaic acid. | |
| - | + | [[Image:Duiril.png]] | |
| - | + | ||
| - | == | + | == Function and Mechanism== |
| + | |||
| + | <scene name='75/756546/Drug/1'>Chlorothiazide </scene> has diuretic and anti-hypertensive properties. Chlorothiazide acts as a diuretic, which means that it inhibits the reabsorption of chloride. This occurs at the distal tubules via the sodium-chloride co-transporter. The result of this is an increased excretion of sodium, chlorine, and water. Chlorothiazide also inhibits the sodium ion transport across renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter. The result of this is an increase in the excretion of potassium using the sodium-potassium exchange system. More specifically, chlorothiazide targets<scene name='75/756546/Solute_carrier_family_12_membe/2'> solute carrier family 12 member 3 </scene> which mediates sodium and chloride reabsorption in the nephron segment to enhance renal sodium reabsorption. As for the anti-hypertensive properties of chlorothiazide the mechanism is not quite as known. It is thought that vasodilation is caused by the activation of calcium-activated potassium channels (KCa) and the inhibition of carbonic anhydrases. <ref name = "one"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 | ||
| + | </ref>. | ||
| + | Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_1/1'>carbonic anhydrase 1</scene> to perform the reversible hydration of carbon dioxide.<scene name='75/756546/Carbonic_anhydrase_2/1'>carbonic anhydrase 2</scene> is also target to perform the reversible hydration of carbon dioxide, regulate the fluid secretion of the anterior chamber of the eye and the intracellular pH in the duodenal upper villous epithelium durin proton-coupled peptide absorption, and stimulate the chloride-bicarbonate exchange activity of SLC26A6. Chlorothiazide targets <scene name='75/756546/Carbonic_anhydrase_4/1'>carbonic anhydrase 4</scene> to perform the reversible hydration of carbon dioxide, stimulate the sodium/bicarbonate transporter activity of SLC4A4, and remove acid overload from the retina and retina epithelium <ref name = "one"> The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880 | ||
| + | </ref>. | ||
| - | Chlorothiazide is a semisynthetic chemical compound known chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide (Figure 1). Its chemical formula is C7H8ClN3O4S2 and its molecular weight is of 298Da. This chemical compound consists of an aromatic ring, benzothiadiazine, a sulfonamide group, and a chloride. It has a melting point of 272 degrees Celsius, a flash point of 302.7 degrees Celcius, a solubility of 60 mg/m in DMSO and less than 1 mg/ml in water, and appears a white crystalline powder. The structure was determined by X-Ray diffraction and was measured at a resolution of 2.1 Angstroms. Cholorothiazide was determined when bound to glutamate receptor 2 through hydrogen bonding between the nitrogen 12 and serine 242B, nitrogen 1 and serines 108B and 108E, and oxygen 17 and glycine 219 E (Figure 2 & 3). Synthesis of chlorothiazide occurs through the reaction between 3 -chloroaniline, chlorosulfonic acid, and ammonia; and its catalyzed by fomaic acid (Figure 4). | ||
<center>[[Image:Duiril.png|thumb|left|200px|Figure 1. Molecule structure of chlorothiazide.]]</center> | <center>[[Image:Duiril.png|thumb|left|200px|Figure 1. Molecule structure of chlorothiazide.]]</center> | ||
Revision as of 23:01, 29 March 2017
Diuril (Chlorothiazide)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 The Metabolomics Innovation Centre. (2016) Chlorothiazide, DrugBank. Retrieved from https://www.drugbank.ca/drugs/DB00880
- ↑ Greene, J.A. (2005) Releasing the flood waters: diuril and the reshaping of hypertension, Bull. Hist. Med. 79, 749-794.
- ↑ 3.0 3.1 Drug.com. (2017) Diuril, Drugs.com. Retrieved from https://www.drugs.com/pro/diuril.html
- ↑ RxList Inc. (2017) Medical definition of diuretic, RxList: The Internet Drug Index. Retrieved from http://www.rxlist.com/script/main/art.asp?articlekey=7103
- ↑ Crawford, J.D., Kennedy, G.C., and Hill, L.E. (1960) Clinical results of treatment of diabetes insipidus with drugs of the chlorothiazide series, N. Engl. J. Med. 262, 737-743.
- ↑ 6.0 6.1 Simon, H. and Zieve, D. (2012) Kidney stones, University of Maryland Medical Center. Retrieved from http://umm.edu/health/medical/reports/articles/kidney-stones
- ↑ AHFS Patient Medication Information. (2017) Chlorothiazide, U.S. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a682341.html
- ↑ The Mayo Clinic Staff. (2017) Idiopathic thrombocytopenic purpura (ITP), Mayo Clinic. Retrieved from http://www.mayoclinic.org/diseases-conditions/idiopathic-thrombocytopenic-purpura/symptoms-causes/dxc-20201224
- ↑ Jaffe, M.O. and Kierland, R. R. (1958) Purpura due to chlorothiazide (diuril), J. Am. Med. Assoc. 168, 2264-2265.