We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
IntronA (Interferon alpha 2b)
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Function == | == Function == | ||
| - | IFNα2b is a naturally secreted protein that is produced by the human body via antigen-presenting cells (APCs) <ref name="two"> doi: 10.1089/107999099313325</ref>. When introduced to the body for treatment, IFNα2b is injected into the body subcutaneously and binds to the surface of cells <ref name="three"> https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf</ref> When this occurs, IFNα2b produces many possible outcomes. This includes inhibiting replication of viruses within virus-infected cells, suppressing cell proliferation, enhancing activity of macrophages, stimulating certain types of enzymes, and increasing lymphocytes’ specific cytotoxicity. | + | IFNα2b is a naturally secreted protein that is produced by the human body via antigen-presenting cells (APCs) <ref name="two"> doi: 10.1089/107999099313325</ref>. When introduced to the body for treatment, IFNα2b is injected into the body subcutaneously and binds to the surface of cells <ref name="three"> https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf</ref>. When this occurs, IFNα2b produces many possible outcomes. This includes inhibiting replication of viruses within virus-infected cells, suppressing cell proliferation, enhancing activity of macrophages, stimulating certain types of enzymes, and increasing lymphocytes’ specific cytotoxicity <ref name="three"> https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf</ref>. Specifically, in viral infections and malignancy the interferon specifically targets CD8+ effector T cells and CD4+ immunomodulatory T cells to amplify the body’s immune response <ref name="two"> doi: 10.1089/107999099313325</ref>. It can also help induce a caspase cascade to activate cell death in virally infected and malignant cells <ref name="two"> doi: 10.1089/107999099313325</ref>. Overall, INFα2b is crucial for activation and regulation of the protective immune response. |
== Structural highlights == | == Structural highlights == | ||
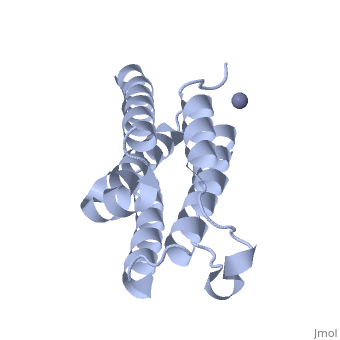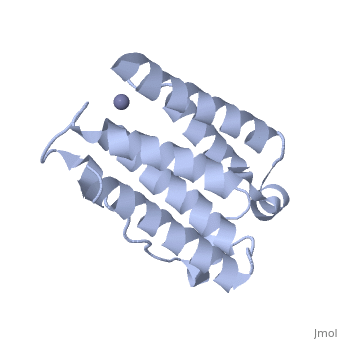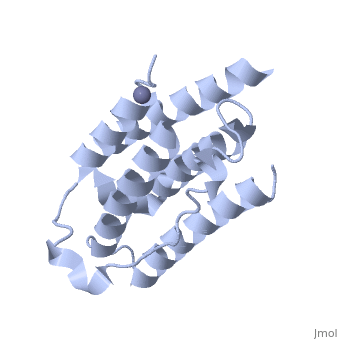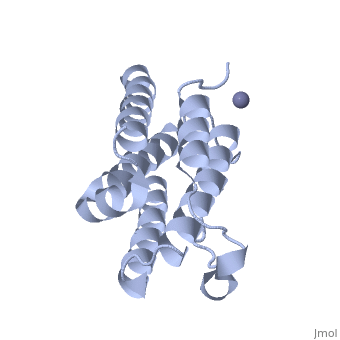
| - | Each IFNα2b monomer consists of five alpha helices and five coiled loops. | + | Each IFNα2b monomer consists of five alpha helices and five coiled loops <ref name="four"> PMID: |
| + | 8994971 </ref>. A zinc molecule is held within each IFNα2b monomer <ref name="four"> PMID: | ||
| + | 8994971 </ref>. There are four significant cysteine residues on IFNα2b that create two disulfide bonds <ref name="one"> doi: 10.1155/2014/970315</ref>. The first disulfide bond is between Cys29 and Cys138 <ref name="one"> doi: 10.1155/2014/970315</ref>. The second disulfide bond is between Cys1 and Cys98 <ref name="one"> doi: 10.1155/2014/970315</ref>. IFNα2b is considered a Type I interferon and recognizes interferon alpha receptors 1 and 2 located on the target protein’s surface <ref name="one"> doi: 10.1155/2014/970315</ref>. Amino acid residues on IFNα2b that participate in binding to IFNAR 1 and 2 are present on one coiled loop and four alpha helices <ref name="one"> doi: 10.1155/2014/970315</ref>. These residues include: Arg22, Leu26, Phe27, Leu30, Lys31, Arg33, His34, Ser68, Thr79, Lys83, Tyr85, Tyr89, Arg120, Lys121, Gln124, Lys131 Glu132, Arg144, and Glu146 <ref name="one"> doi: 10.1155/2014/970315</ref>. | ||
== Mechanism == | == Mechanism == | ||
There are several pathways in that IFNα2b has an effect on the target cell <ref name="one"> doi: 10.1155/2014/970315</ref>. These pathways include the caspase cascade and the JAK-STAT pathway <ref name="one"> doi: 10.1155/2014/970315</ref> <ref name="two"> doi: 10.1089/107999099313325</ref>. The caspase cascade results in apoptosis, thereby participating in both anti-viral and anti-cancer mechanisms <ref name="two"> doi: 10.1089/107999099313325</ref>. Once IFNα2b binds to either IFNAR 1 or 2 receptors, cytochrome c and tumor necrosis alpha factor trigger the caspase cascade, which in turn signals apoptosis <ref name="one"> doi: 10.1155/2014/970315</ref>. In the binding of IFNα2b to an IFNAR receptor, the protein JAK (a tyrosine kinase) is activated <ref name="one"> doi: 10.1155/2014/970315</ref>. JAK is phosphorylated and in turn phosphorylates the IFNAR receptors <ref name="one"> doi: 10.1155/2014/970315</ref>. The IFNAR receptors bind to STAT proteins resulting in a cascade pathway that signals the release of antiviral proteins <ref name="one"> doi: 10.1155/2014/970315</ref>. | There are several pathways in that IFNα2b has an effect on the target cell <ref name="one"> doi: 10.1155/2014/970315</ref>. These pathways include the caspase cascade and the JAK-STAT pathway <ref name="one"> doi: 10.1155/2014/970315</ref> <ref name="two"> doi: 10.1089/107999099313325</ref>. The caspase cascade results in apoptosis, thereby participating in both anti-viral and anti-cancer mechanisms <ref name="two"> doi: 10.1089/107999099313325</ref>. Once IFNα2b binds to either IFNAR 1 or 2 receptors, cytochrome c and tumor necrosis alpha factor trigger the caspase cascade, which in turn signals apoptosis <ref name="one"> doi: 10.1155/2014/970315</ref>. In the binding of IFNα2b to an IFNAR receptor, the protein JAK (a tyrosine kinase) is activated <ref name="one"> doi: 10.1155/2014/970315</ref>. JAK is phosphorylated and in turn phosphorylates the IFNAR receptors <ref name="one"> doi: 10.1155/2014/970315</ref>. The IFNAR receptors bind to STAT proteins resulting in a cascade pathway that signals the release of antiviral proteins <ref name="one"> doi: 10.1155/2014/970315</ref>. | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
== Disease == | == Disease == | ||
| Line 26: | Line 27: | ||
''Hepatitis C'' | ''Hepatitis C'' | ||
| - | Hepatitis C (HCV) can range from acute to chronic conditions and progression occurs in more than half of these cases. | + | Hepatitis C (HCV) can range from acute to chronic conditions and progression occurs in more than half of these cases <ref name="five"> doi: 10.1056/NEJMoa011232</ref>. HCV attacks the liver tissues causing inflammation and cirrhosis, which can lead to chronic liver disease <ref name="five"> doi: 10.1056/NEJMoa011232</ref>. IFNα2b is used to treat the acute stage of HVC to prevent progression into the chronic disease <ref name="five"> doi: 10.1056/NEJMoa011232</ref>. IFNα2b overall reduces the viral amplification and allows T-cells to effectively respond to the infection <ref name="six"> Doi: 10.1016/j.virol.2004.04.031</ref>. |
''Hepatitis B'' | ''Hepatitis B'' | ||
| - | IFNα2b treats chronic Hepatitis B (HBV) by interfering with viral DNA synthesis and enhancing the cellular immune response.7 The cytokine enhances T-cell and natural killer cell activity by interacting with the infected cell’s surface. | + | IFNα2b treats chronic Hepatitis B (HBV) by interfering with viral DNA synthesis and enhancing the cellular immune response.7 The cytokine enhances T-cell and natural killer cell activity by interacting with the infected cell’s surface <ref name="seven"> doi: 10.1016/j.cld.2007.08.010</ref>. IFNα2b also activates antiviral enzymes to inhibit proliferation of HBV <ref name="seven"> doi: 10.1016/j.cld.2007.08.010</ref>. |
'''Cancer''' | '''Cancer''' | ||
| Line 36: | Line 37: | ||
''Multiple Myeloma'' | ''Multiple Myeloma'' | ||
| - | IFNα2b has been shown to prolong patients with multiple myeloma. | + | IFNα2b has been shown to prolong patients with multiple myeloma <ref name="eight"> doi: 10.1038/sj.leu.2401039</ref>. Multiple myeloma is diagnosed when multiple clones of a specific plasma cell are found or when apparent genetic mutations are affecting the cell’s normal functions <ref name="eight"> doi: 10.1038/sj.leu.2401039</ref>. IFNα2b therapy causes tumor stabilization and prolongation of the aggressive stage of multiple myeloma with maintained therapy <ref name="eight"> doi: 10.1038/sj.leu.2401039</ref>. |
''Hairy cell Leukemia'' | ''Hairy cell Leukemia'' | ||
| - | IFNα2b is a major treatment for patients with hairy cell leukemia <ref name="one"> doi: 10.1155/2014/970315</ref>. The disease is characterized by B cells in the blood, bone marrow, and spleen <ref name="one"> doi: 10.1155/2014/970315</ref>. Patients who receive the therapeutic treatment experience partial to complete remission <ref name="one"> doi: 10.1155/2014/970315</ref>. The treatment is especially effective because there is rarely relapse of the cancer with consistent therapy and mild side effects <ref name="one"> doi: 10.1155/2014/970315</ref>. The presence of malignant cells decreases, predominantly in the bone marrow, and hematologic levels normalized. | + | IFNα2b is a major treatment for patients with hairy cell leukemia <ref name="one"> doi: 10.1155/2014/970315</ref>. The disease is characterized by B cells in the blood, bone marrow, and spleen <ref name="one"> doi: 10.1155/2014/970315</ref>. Patients who receive the therapeutic treatment experience partial to complete remission <ref name="one"> doi: 10.1155/2014/970315</ref>. The treatment is especially effective because there is rarely relapse of the cancer with consistent therapy and mild side effects <ref name="one"> doi: 10.1155/2014/970315</ref>. The presence of malignant cells decreases, predominantly in the bone marrow, and hematologic levels normalized <ref name="nine"> doi: 10.1056/NEJM198401053100104</ref> |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | |||
| - | Ningrum A. R., & Ratih. (2014). Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica, 2014, 970315. doi: 10.1155/2014/970315 | ||
| - | |||
| - | Tompkins, W. A. (1999). Immunomodulation and Therapeutic Effects of the Oral Use of Interferon-alpha: Mechanism of Action. Journal of Interferon & Cytokine Research, 19(8), 817–828. doi: 10.1089/107999099313325 | ||
| - | |||
| - | Merck. (n.d.). Product information intron A interferon alfa-2b, recombinant for injection. Retrieved from https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf. | ||
| - | |||
| - | Radhakrishnan, R., Walter, L. J., Hruza, A., Reichert, P., Trotta, P. P., Nagabhushan, T. L., & Walter, M. R. (1996). Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure (London, England : 1993), 4(12), 1453–63. | ||
| - | |||
| - | Jaeckel, E., Cornberg, M., Wedemeyer, H., Santantonio, T., Mayer, J., Zankel, M., Pastore, G., Dietrich, M., Trautwein, C. & Manns, M. P. (2001). Treatment of Acute Hepatitis C with Interferon Alfa-2b. New England Journal of Medicine, 345(20), 1452–1457. doi: 10.1056/NEJMoa011232 | ||
| - | |||
| - | Guo, J.T., Sohn, A.J., Zhu, Q. & Seeger, C. (2004). Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology, 35 (1), 71-81. Doi: 10.1016/j.virol.2004.04.031 | ||
| - | |||
| - | Asselah, T., Lada, O., Moucari, R., Le Martinot, M., Boyer, N., & Marcellin, P. (n.d.). Interferon Therapy for Chronic Hepatitis B. doi: 10.1016/j.cld.2007.08.010 | ||
| - | |||
| - | Bladé, J., San Miguel, J., Escudero, M., Fontanillas, M., Besalduch, J., Gardella, S., Arias, J., Garcia-Conte, J., Carnero, M., Marti, J.M., Rozman, C., Estape, J. & Montserrat, E. (1998). Maintenance treatment with interferon alpha-2b in multiple myeloma:a prospective randomized study from PETHEMA ( Program for the Study and Treatment of Hematological Malignancies , Spanish Society of Hematology ). Leukemia, 12, 1144–1148. doi: 10.1038/sj.leu.2401039 | ||
| - | |||
| - | Quesada, J. R., Reuben, J., Manning, J. T., Hersh, E. M., & Gutterman, J. U. (1984). Alpha Interferon for Induction of Remission in Hairy-cell Leukemia. The New England Journal of Medicine Downloaded from Nejm.org at JAMES MADISON UNIV on, 310(1), 15–18. | ||
Revision as of 06:30, 30 March 2017
An overview of IntronA (Interferon alpha 2b)
IntronA, also known as interferon alpha-2b (IFNα2b), is a drug used in antiviral and anti-tumor therapeutic treatments [1]. IFNα2b is classified as a cytokine, a secreted protein that stimulates the immune system, and is produced by T-cells in order to hinder viral infections, cancer, bacteria, or other pathogens in humans [1].
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 Asmana Ningrum R. Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica (Cairo). 2014;2014:970315. doi: 10.1155/2014/970315. Epub 2014 Mar, 10. PMID:24741445 doi:http://dx.doi.org/10.1155/2014/970315
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 4.0 4.1 4.2 4.3 4.4 Tompkins WA. Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res. 1999 Aug;19(8):817-28. PMID:10476925 doi:http://dx.doi.org/10.1089/107999099313325
- ↑ 5.0 5.1 https://www.merck.com/product/usa/pi_circulars/i/intron_a/intron_a_pi.pdf
- ↑ 6.0 6.1 Radhakrishnan R, Walter LJ, Hruza A, Reichert P, Trotta PP, Nagabhushan TL, Walter MR. Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure. 1996 Dec 15;4(12):1453-63. PMID:8994971
- ↑ 7.0 7.1 7.2 Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, Pastore G, Dietrich M, Trautwein C, Manns MP. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001 Nov 15;345(20):1452-7. PMID:11794193 doi:http://dx.doi.org/10.1056/NEJMoa011232
- ↑ Guo JT, Sohn JA, Zhu Q, Seeger C. Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology. 2004 Jul 20;325(1):71-81. PMID:15231387 doi:http://dx.doi.org/10.1016/j.virol.2004.04.031
- ↑ 9.0 9.1 Asselah T, Lada O, Moucari R, Martinot M, Boyer N, Marcellin P. Interferon therapy for chronic hepatitis B. Clin Liver Dis. 2007 Nov;11(4):839-49, viii. PMID:17981231 doi:http://dx.doi.org/10.1016/j.cld.2007.08.010
- ↑ 10.0 10.1 10.2 Blade J, San Miguel JF, Escudero ML, Fontanillas M, Besalduch J, Gardella S, Arias J, Garcia-Conde J, Carnero M, Marti JM, Rozman C, Estape J, Montserrat E. Maintenance treatment with interferon alpha-2b in multiple myeloma: a prospective randomized study from PETHEMA (Program for the Study and Treatment of Hematological Malignancies, Spanish Society of Hematology). Leukemia. 1998 Jul;12(7):1144-8. doi: 10.1038/sj.leu.2401039. PMID:9665202 doi:http://dx.doi.org/10.1038/sj.leu.2401039
- ↑ Quesada JR, Reuben J, Manning JT, Hersh EM, Gutterman JU. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984 Jan 5;310(1):15-8. PMID:6689734 doi:http://dx.doi.org/10.1056/NEJM198401053100104