Sandbox Reserved 1070
From Proteopedia
| Line 5: | Line 5: | ||
== Biological Function == | == Biological Function == | ||
| - | Diguanylate cyclases, class 2 transferase enzymes, catalyze the production of cyclic di-GMP | + | Diguanylate cyclases, class 2 transferase enzymes, catalyze the production of cyclic dimeric-GMP (c-di-GMP), important to signal transduction as a second messenger. Through the synthesis of cyclic di-GMP from two substrate GTP molecules, utilizing enzymes including DgcZ, signals are communicated throughout the bacteria. C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc), a polysaccharide required for ''E. coli'' biofilm production. This biofilm allows ''E. coli'' to adhere to extracellular surfaces. The DgcZ protein is composed of two domains: the catalytic glycine-glycine-glutamate-glutamate-phenylalanine (GGEEF) domain responsible for synthesizing c-di-GMP and the regulatory chemoreceptor zinc binding (CZB) domain comprising two zinc binding sites. DgcZ binds zinc with sub-femtomolar affinity. When zinc is bound, the CZB and GGEEF domains adopt conformations that inhibit DgcZ function. |
== Structural Overview == | == Structural Overview == | ||
Revision as of 00:51, 31 March 2017
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Contents |
DgcZ from E. coli
|
Biological Function
Diguanylate cyclases, class 2 transferase enzymes, catalyze the production of cyclic dimeric-GMP (c-di-GMP), important to signal transduction as a second messenger. Through the synthesis of cyclic di-GMP from two substrate GTP molecules, utilizing enzymes including DgcZ, signals are communicated throughout the bacteria. C-di-GMP is a second messenger in the production of poly-β-1,6-N-acetylglucosamine (poly-GlcNAc), a polysaccharide required for E. coli biofilm production. This biofilm allows E. coli to adhere to extracellular surfaces. The DgcZ protein is composed of two domains: the catalytic glycine-glycine-glutamate-glutamate-phenylalanine (GGEEF) domain responsible for synthesizing c-di-GMP and the regulatory chemoreceptor zinc binding (CZB) domain comprising two zinc binding sites. DgcZ binds zinc with sub-femtomolar affinity. When zinc is bound, the CZB and GGEEF domains adopt conformations that inhibit DgcZ function.
Structural Overview
The CZB domain is common to many bacterial lineages, appearing most commonly in bacterial chemoreceptors involved in chemotaxis. The second most common group of CZB domains is that of DgcZ homologs. [1]. The domain has an important role in signal transduction of bacteria[1]. 30 small bacterial proteins of family PRK0984 from differing strands of E. coli contain a CZB domain N-terminal to a GGDEF domain[1].
Mechanism of Action
Diguanylate cyclases only function efficiently as dimers, to bind both GGDEF domains holding the substrates. The presence of Zinc disrupts the ability of the two domains to overlap.
Zinc Ligand(s)
Most cells possess efficient Zinc uptake systems, as Zinc is a reactive Lewis Acid. Zinc binds incredibly tightly to this enzyme at subfemtomolar concentrations. The Zinc co-purified with the protein.
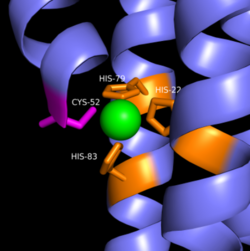
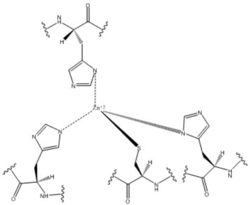
Zinc Binding Site
Zinc allosterically inhibits the activity of enzyme DgcZ through an allosteric binding site located on the CZB domain. The inhibition prevents regulation of GGDEF domain function, the location of the active site. The CZB domain is folded into four anti-parallel α-helices as a 2-fold symmetric homodimer, with the N-terminus on the helix 𝝰4. The allosteric binding site includes amino acids, H22 of 𝝰1, C52 of 𝝰2, and H79 and H83 of 𝝰3, that span three of the four alpha helices of the CZB domain coordinating the Zinc residue. The cysteine residue is not essential for Zinc binding, as when the cysteine residue was mutated to alanine, the catalytic activity of the enzyme increased. The cysteine does however plays a role in coordinating the metal to the allosteric site. Using EDTA, Zinc can be removed from the CZB domain. The zinc has higher affinity for EDTA than CZB when EDTA concentration is higher than the concentration of DgcZ. Upon removing Zinc, the 𝝰1 helix straightens, burying nonpolar side-chain residues and subsequently increasing the activity of DgcZ. Activity increases without Zinc due to activation of poly-GlcNAc production and biofilm formation, and maximal cyclic di-GMP production.
Other Ligands
Binding Site
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
<Jenny Draper, K. Karplus, K. Ottemann. Identification of a Chemoreceptor Zinc-Binding Domain Common to Cytoplasmic Bacterial Chemoreceptors. Journal of Bacteriology. Vol. 193, No. 17. 4338-4345. (2011).>