User:Natalie Van Ochten/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==General Structure== | ==General Structure== | ||
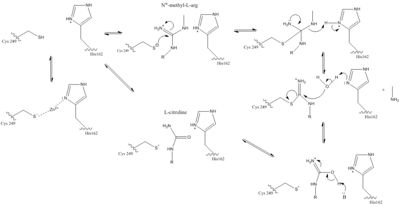
| - | DDAH’s <scene name='69/694225/Secondary_structure_colored/3'>secondary structure</scene> has a propeller-like fold which is characteristic of the superfamily of <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine:glycine_amidinotransferase L-arginine/glycine amidinotransferases]</span> <ref name="humm">Humm A, Fritsche E, Mann K, Göhl M, Huber R. Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue. Biochemical Journal. 1997 March 15;322(3):771-776. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1218254/ 9148748]</span> doi:<span class="plainlinks">[http://www.biochemj.org/content/322/3/771 10.1042/bj3220771]</span></ref>. This five-stranded propeller contains five repeats of a ββαβ motif <ref name="frey" />. This motif consists of two <span class="plainlinks">[https://en.wikipedia.org/wiki/Beta_sheet beta-sheets]</span> that are anti-parallel, an <span class="plainlinks">[https://en.wikipedia.org/wiki/Alpha_helix alpha helix]</span>, and another beta-sheet that is parallel to the second beta-sheet in the motif. These motifs in DDAH form a channel in the center of the protein structure. Lys174 and Glu77 form a <scene name='75/752351/Ddah_salt_bridge/3'>salt bridge</scene> in the channel that forms the bottom of the active site for the protein. One side of the channel is a | + | DDAH’s <scene name='69/694225/Secondary_structure_colored/3'>secondary structure</scene> has a propeller-like fold which is characteristic of the superfamily of <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine:glycine_amidinotransferase L-arginine/glycine amidinotransferases]</span> <ref name="humm">Humm A, Fritsche E, Mann K, Göhl M, Huber R. Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue. Biochemical Journal. 1997 March 15;322(3):771-776. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1218254/ 9148748]</span> doi:<span class="plainlinks">[http://www.biochemj.org/content/322/3/771 10.1042/bj3220771]</span></ref>. This five-stranded propeller contains five repeats of a ββαβ motif <ref name="frey" />. This motif consists of two <span class="plainlinks">[https://en.wikipedia.org/wiki/Beta_sheet beta-sheets]</span> that are anti-parallel, an <span class="plainlinks">[https://en.wikipedia.org/wiki/Alpha_helix alpha helix]</span>, and another beta-sheet that is parallel to the second beta-sheet in the motif. These motifs in DDAH form a <scene name='75/752351/Ddah_water_pore/2'>channel</scene> in the center of the protein structure. Lys174 and Glu77 form a <scene name='75/752351/Ddah_salt_bridge/3'>salt bridge</scene> in the channel that forms the bottom of the active site for the protein. One side of the channel is a water-filled pore, whereas the other side is the active site cleft that is defined by a short loop region and alpha helical structures <ref name="frey" />. |
===Lid Region=== | ===Lid Region=== | ||
Revision as of 01:26, 31 March 2017
Dimethylarginine Dimethylaminohydrolase
| |||||||||||
References
- ↑ 1.0 1.1 Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:17933965 doi:10.1152/ajpheart.00998.2007
- ↑ 2.0 2.1 2.2 Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:14664901 doi:10.1016/S1567-5688(03)00032-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:16698551 doi:10.1016/j.str.2006.03.006
- ↑ Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:23097221 doi:10.1002/path.4127
- ↑ Humm A, Fritsche E, Mann K, Göhl M, Huber R. Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue. Biochemical Journal. 1997 March 15;322(3):771-776. PMID:9148748 doi:10.1042/bj3220771
- ↑ 6.0 6.1 6.2 Rasheed M, Richter C, Chisty LT, Kirkpatrick J, Blackledge M, Webb MR, Driscoll PC. Ligand-dependent dynamics of the active site lid in bacterial Dimethyarginine Dimethylaminohydrolase. Biochemistry. 2014 Feb 18;53:1092-1104. PMCID:PMC3945819 doi:10.1021/bi4015924
- ↑ 7.0 7.1 Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of Dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006 May 2;45(17):5618-5630. PMID:16634643 doi:10.1021/bi052595m
- ↑ 8.0 8.1 Pace NJ, Weerpana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014 June;4(2):419-434. PMCID:4101490 doi:10.3390/biom4020419
- ↑ Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:10979862 doi:10.1016/S0165-6147(00)01499-1
- ↑ Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:14975444 doi:10.1016/j.freeradbiomed.2003.11.011
- ↑ Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:9883748
- ↑ Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:12461516 doi:10.1038/nrd960