Aspirin effects on COX aka PGHS
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
While the use of aspirin goes back to ancient times, the mechanism of how aspirin relieves pain is relatively modern. In 1971, John Vane discovered that aspirin works by inhibiting the synthesis of prostaglandins <ref>PMID:5284360</ref>, and he recieved a Nobel Prize for this work in 1982 <ref>http://nobelprize.org/nobel_prizes/medicine/laureates/1982/vane-lecture.html</ref>. In 1975 Roth, Standford, and Majerus identified prostaglandin H2 synthase, also referred to as cyclooxygenase (COX), as the enzyme that aspirin inhibits<ref>PMID 810797</ref>. | While the use of aspirin goes back to ancient times, the mechanism of how aspirin relieves pain is relatively modern. In 1971, John Vane discovered that aspirin works by inhibiting the synthesis of prostaglandins <ref>PMID:5284360</ref>, and he recieved a Nobel Prize for this work in 1982 <ref>http://nobelprize.org/nobel_prizes/medicine/laureates/1982/vane-lecture.html</ref>. In 1975 Roth, Standford, and Majerus identified prostaglandin H2 synthase, also referred to as cyclooxygenase (COX), as the enzyme that aspirin inhibits<ref>PMID 810797</ref>. | ||
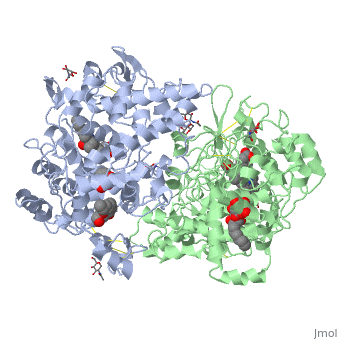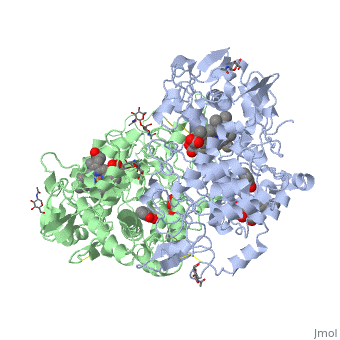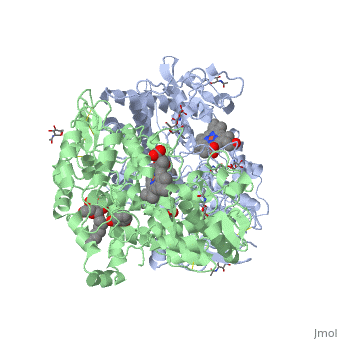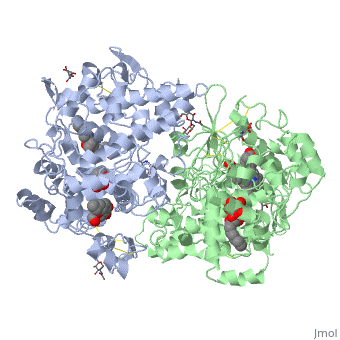
| - | PGHS contains two identical polypeptide chains, shown in blue and green in the box at the right. The enzyme catalyzes two reactions: first, the <scene name='Aspirin_effects_on_COX_aka_PGHS/Heme_1/5'>heme</scene> reacts with the oxygen to create a peroxide at <scene name='37/377115/Aa_tyr_heme/1'>tyrosine 385</scene>; in the second reaction, the peroxide is added to the double bond in | + | PGHS contains two identical polypeptide chains, shown in blue and green in the box at the right. The enzyme catalyzes two reactions: first, the <scene name='Aspirin_effects_on_COX_aka_PGHS/Heme_1/5'>heme</scene> reacts with the oxygen to create a peroxide at <scene name='37/377115/Aa_tyr_heme/1'>tyrosine 385</scene>; in the second reaction, the peroxide is added to the double bond in arachadonic acid. |
Aspirin works by transferring the acetyl group (CH3COO-) of aspirin to the OH of the <scene name='37/377115/Ser_530_with_label/1'>Ser 530</scene>. When this residue is acetylated, arachidonic acid can no longer be bound because there is no longer room for it to fit in the enzyme. Since the acetylation cannot be undone, this is a "suicide" inhibitor--the inhibition cannot be undone, and the enzyme is dead. | Aspirin works by transferring the acetyl group (CH3COO-) of aspirin to the OH of the <scene name='37/377115/Ser_530_with_label/1'>Ser 530</scene>. When this residue is acetylated, arachidonic acid can no longer be bound because there is no longer room for it to fit in the enzyme. Since the acetylation cannot be undone, this is a "suicide" inhibitor--the inhibition cannot be undone, and the enzyme is dead. | ||
Revision as of 20:14, 17 April 2017
ASPIRIN'S ANTI-INFLAMMATORY EFFECTS UPON PROSTAGLANDIN H2 SYNTHASE (CYCLOOXYGENASE)
| |||||||||||
About this Structure
1PTH is a 2 chains structure of sequences from Ovis aries. The May 2001 RCSB PDB Molecule of the Monthby David S. Goodsell features Cyclooxygenase. [1]. Full crystallographic information is available from OCA.
References
<- ↑ http://www.bellaonline.com/articles/art8190.asp
- ↑ Quinque Mayer R, Mayer M. Mayer R, Mayer M. Biological salicyltherapy with cortex salicus [Weidenrinde]. Pharmazie 1949;4:77-81.
- ↑ Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232-5. PMID:5284360
- ↑ http://nobelprize.org/nobel_prizes/medicine/laureates/1982/vane-lecture.html
- ↑ Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073-6. PMID:810797